(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a genitourinary cancers poster session. Professor Karim Fizazi presented updated results of the phase II CYPIDES trial of opevesostat (ODM-208/MK-5684), an oral CYP11A1 inhibitor, in metastatic castration-resistant prostate cancer (mCRPC) patients.
Opevesostat (ODM-208/MK-5684) inhibits the production of all adrenal steroid hormones and precursors. Initial phase I and II results demonstrated frequent PSA responses in heavily pre-treated mCRPC patients, especially in the presence of activating ligand-binding domain (AR-LBD) mutations. Activating AR-LBD mutations occur in 20–25% of patients previously treated with novel hormonal agents and are associated with resistance to such treatment. In this report, Dr. Fizazi presented updated results from the phase II trial in late-stage patients with mCRPC, both with and without AR-LBD mutations.
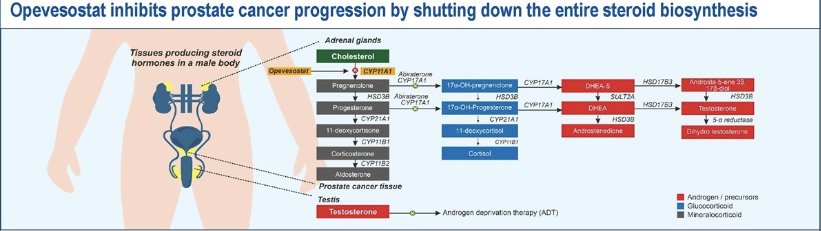
This was an open label, non-randomized trial. Patients received opevesostat 5 mg twice daily + supportive adrenal therapy (dexamethasone 1–1.5 mg and fludrocortisone 0.1 mg) along with ADT until disease progression.
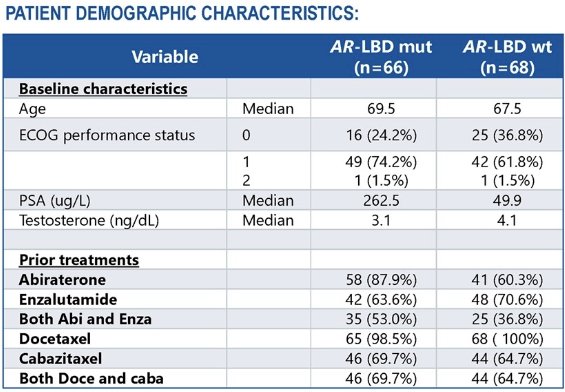
Eligible participants were mCRPC patients with disease progression following ≥1 novel hormonal agent and ≥1 taxane. A total of 134 patients were enrolled, of whom 66 had AR-LBD mutations. AR-LBD status was determined by the Guardant 360 ctDNA assay. Participants were recruited from clinical sites in France, Finland, the USA, and the UK. The median study follow-up was 7.1 months (data cut-off: January 23, 2024).
Baseline patient characteristics are summarized below. Patients without AR-LBD mutations were less likely to have received both abiraterone and enzalutamide and had lower median PSA values at baseline. These differences could reflect patient enrolment during different time periods and conditions favoring the development of AR-LBD mutations.
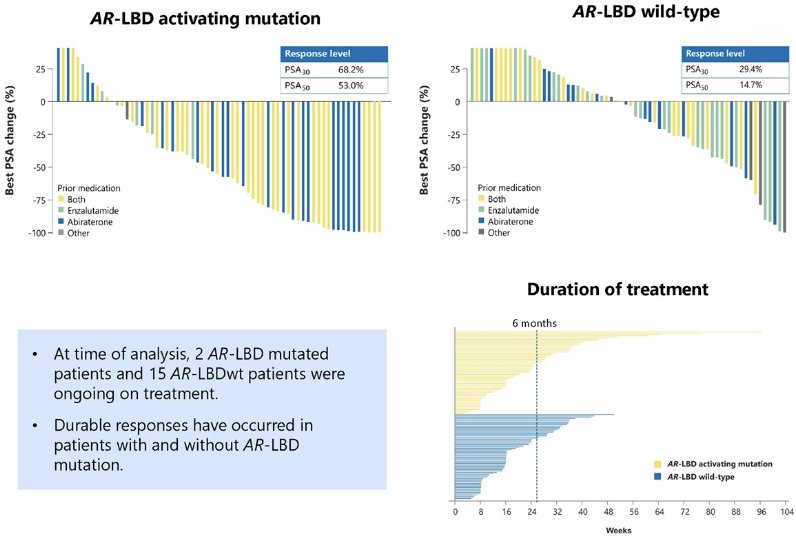
In patients with AR-LBD activating mutations, a PSA50 response was observed in 53% of patients. Conversely, in the AR-LBD wild-type patients, a PSA50 response was observed in 15%. PSA30 responses were observed in 68% and 29% of patients, respectively.
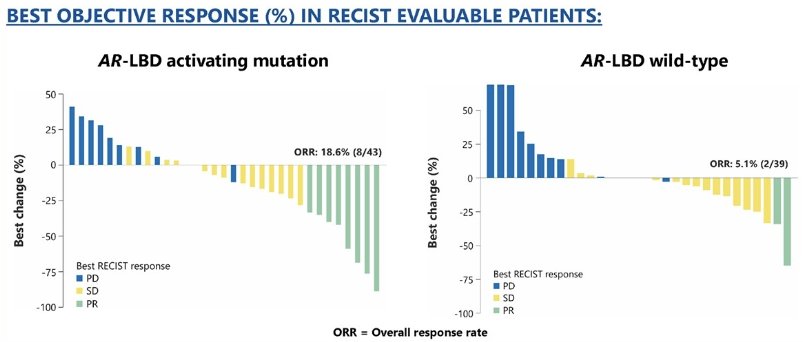
The objective response rates in the AR-LBD mutated and wild type patients were 19% and 5%, respectively.
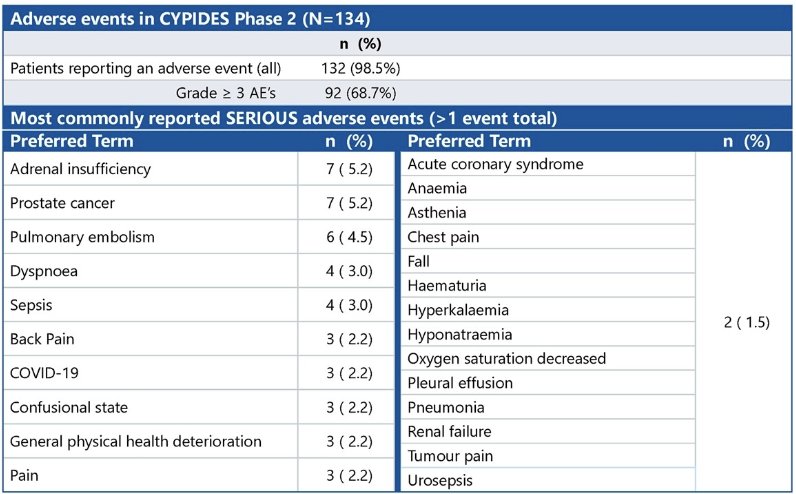
From a safety standpoint, almost all patients had a reported adverse event during study treatment (98.5%), although 86% were grade 1–2 in severity. Minor signs/symptoms of inadequate hormone replacement (e.g., electrolyte disturbances) were common, but adrenal insufficiency requiring hospital admission was infrequent.
Dr. Fizazi concluded that following opevesostat treatment in men with extensively pre-treated mCRPC:
- PSA and RECIST (soft-tissue) responses occurred in men without AR-LBD mutations but were more frequent in those with AR-LBD mutations.
- The number of men with prolonged disease control (>6 months) appears similar with and without AR-LBD mutations, with data still immature.
- The reported adverse events of opevesostat were clinically manageable. The rate of serious adrenal insufficiency was low on steroid replacement therapy.
- Randomized phase III studies of opevesostat in mCRPC are underway.
Presented by: Karim Fizazi, MD, PhD, Professor, Department of Medicine, Institut Gustave Roussy, Paris, France
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


