(UroToday.com) The 2024 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Michael Hofman discussing Prostate Cancer Working Group 4 (PCWG4) preliminary criteria using serial PSMA PET/CT for response evaluation. The PCWG3 consensus guidelines have proven a robust set of imaging endpoints incorporated into most prostate cancer trials. 68Ga-PSMA-11, 18F-DCFPyL, and 18F-rhPSMA-7.3 PET/CT are all FDA approved, and recommended for staging and biochemical recurrence. Further, the randomized ProPSMA study demonstrated accuracy exceeding CT and bone scintigraphy.1 An expert committee of clinical investigators (PCWG4) met in 2023 to consider preliminary imaging criteria that incorporated PSMA PET/CT response assessments. Proposed criteria described complete and partial response, stable disease, and progressive disease. Dr. Hofman and colleagues sought to determine reader reproducibility and the clinical correlation of these categories using a trial dataset that incorporated 12-weekly PSMA PET/CT alongside standard CT / bone scan (PCWG3) assessments.
Five experienced nuclear medicine specialists independently reviewed all available PSMA PET/CT scans from the PRINCE trial (NCT03658447; 177Lu-PSMA-617 + pembrolizumab) blinded to conventional imaging response, according to draft PCWG4 criteria. Complete response was defined by no PSMA-positive lesions. Progressive disease was defined by two new PSMA-positive lesions (not changes in intensity of uptake or SUV) with confirmation required on the 8-12 week scan, but not subsequently:
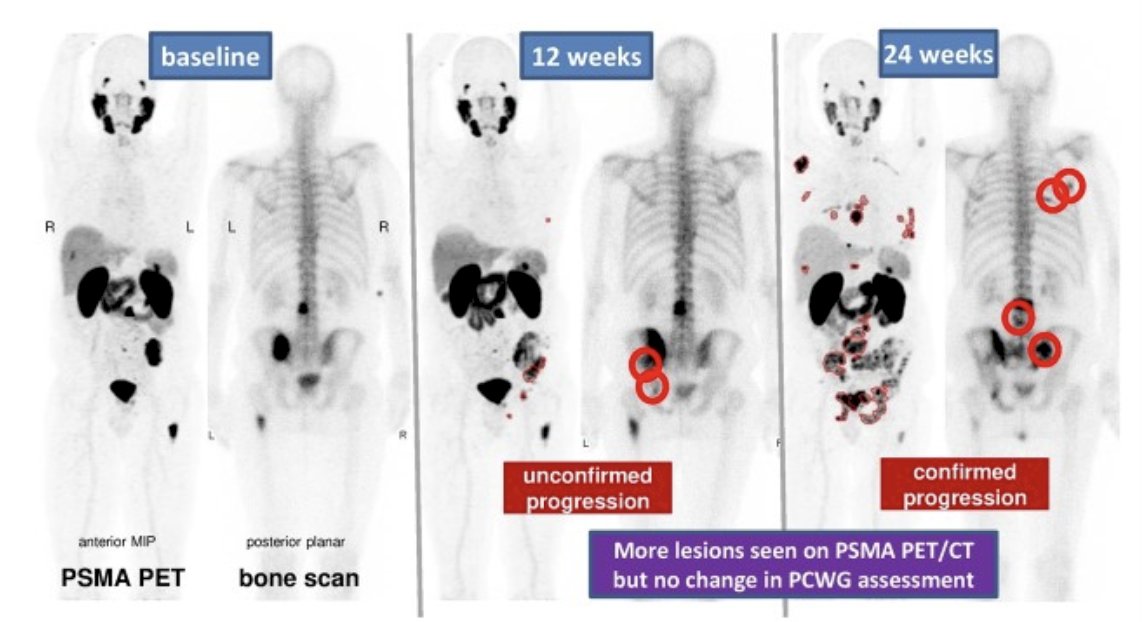
Reporter agreement was measured using multilevel Fleiss Kappa. Radiographic progression-free survival using PCWG3 and PCWG4 criteria were described and correlated with overall survival using interactive multiple imputation for bivariate censored time-to-event data.
There were 36 patients with serial PSMA PET/CT and CT/bone scan analyzed (142 paired scans), including 9 that had RECIST measurable disease. Reporter agreement of PSMA PET/CT response was substantial (0.69, 95% CI: 0.61 - 0.76) and almost perfect (0.90, 95% CI: 0.85 -0.96) for level of response (complete response, partial response, stable disease, progressive disease) or progression (progressive disease vs non-progressive disease), respectively. PCWG4 provided a level of response (2 complete responses, 7 partial responses, 14 stable disease) when PCWG3 best overall response was defined as non-progressive disease:
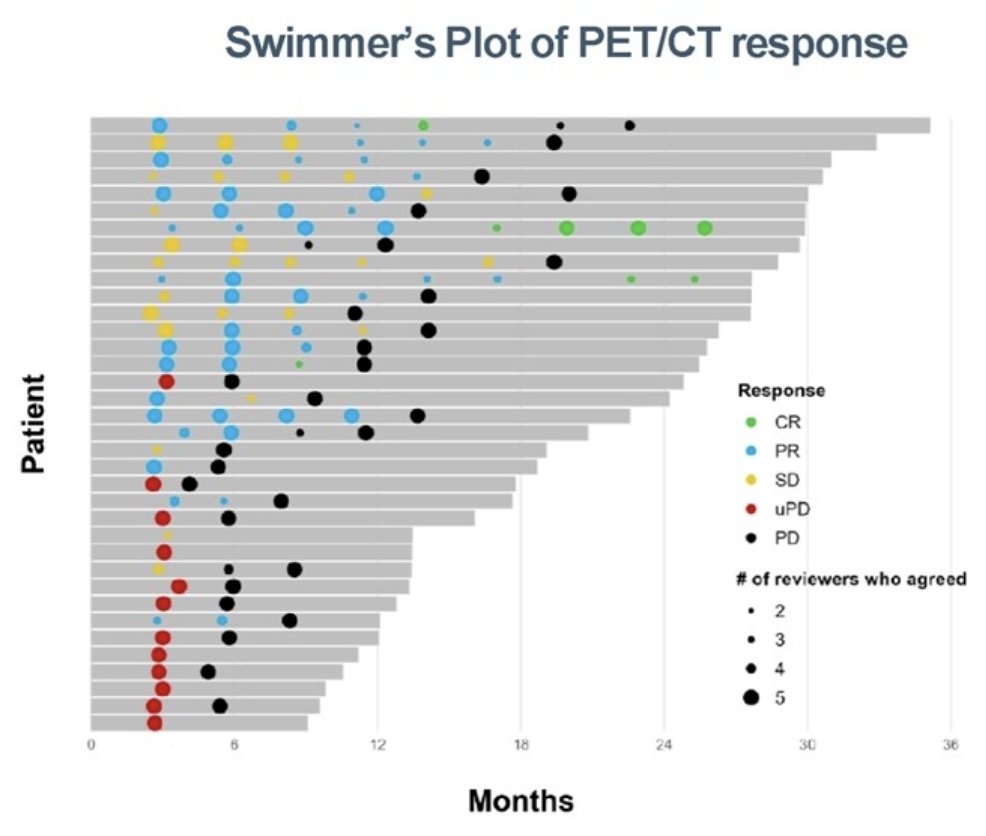
PCWG4 detected imaging defined progression earlier than PCWG3 with a median radiographic progression free survival of 9.4 (5.6 - 13.7) months compared to 19.9 (8.2 – not evaluable) months:
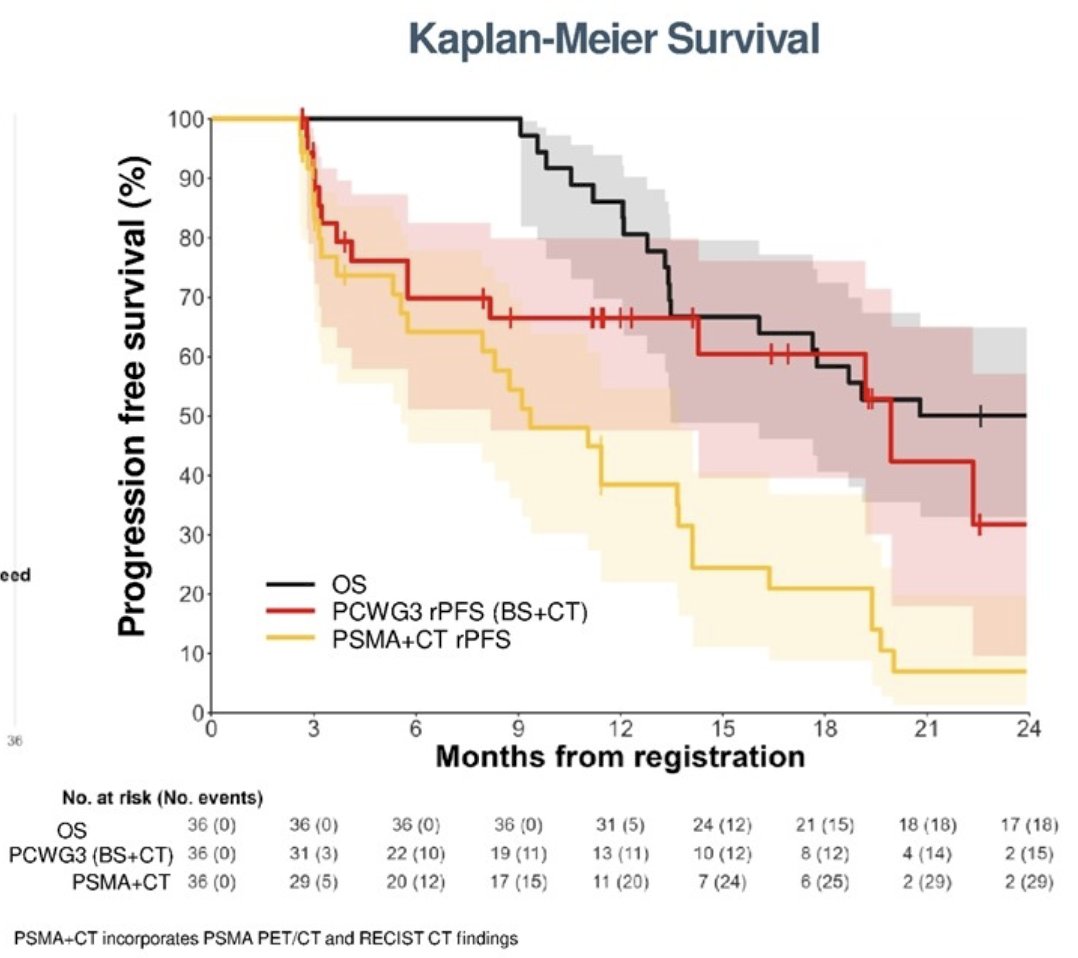
Correlation between PCWG4 and PCWG3 and overall survival was k = 0.86 (95% CI: 0.71 - 0.94) and k = 0.79 (95% CI: 0.58 - 0.91), respectively.
Similar to PCWG3, interpretation of PSMA PET/CT in PCWG4 involve visual criteria that can be widely adopted without need for proprietary software or hardware. Changes in SUV do not define progression or response, and new lesions define progression. Contrast enhanced CT with RECIST are still incorporate with PSMA PET/CT findings replacing bone scan to define progression and response. PCWG4 definitions for PSMA PET/CT progression and response have been informed and evolved since this work and will be published shortly. Quantitative PET parameters (ie. SUVmean, tumor volume) are considered investigational, and central collection as part of trials for analysis is recommended. Finally, limitations of this analysis include the small patient cohort and use of preliminary criteria, which has since undergone revision. The Kaplan Meier plots presented compares “pure imaging” progression and does not consider clinical progression.
Dr. Hofman concluded his presentation discussing Prostate Cancer Working Group 4 preliminary criteria using serial PSMA PET/CT for response evaluation with the following take-home points:
- Agreement of PSMA PET/CT response was substantial for all levels of response and almost perfect for progressive disease vs non-progressive disease
- The criteria evaluated enable evaluation of level of response (complete response, partial response, stable disease) in patients with non-progressive disease by PCWG3 criteria
- These criteria detected progression earlier than PCWG3 that correlated well with overall survival
- These criteria warrant consideration for PCWG4, and further validation work in a larger cohort is being undertaken
Presented by: Michael S. Hofman, MBBS (Hons), FRACP, FAANMS, Professor of Molecular Imaging, The University of Melbourne, and Nuclear Medicine Physician in the Centre for Cancer Imaging at the Peter MacCallum Cancer Centre in Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
Related Content: PRINCE Trial Highlights Early Detection of Prostate Cancer Progression with PSMA PET-CT - Michael Hofman

