(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a genitourinary cancers poster session. Dr. Martina Buffoni presented the results of the BonEnza trial that evaluated total and regional changes in body composition in metastatic hormone-sensitive prostate cancer (mHSPC) patients randomized to receive androgen deprivation therapy (ADT) and enzalutamide +/- zoledronic acid.
The use of ADT has been demonstrated to reduce lean body mass while increasing fat body mass, leading to an increased risk of sarcopenic obesity. To date, the additional effects of androgen receptor pathway inhibitors (ARPIs) have been poorly investigated in mHSPC patients.
BonEnza (NCT03336983) is a prospective phase II trial of mHSPC patients who were randomized to receive ADT + enzalutamide +/- zoledronic acid. Lean body mass and fat body mass were evaluated in different body regions by dual-energy x-ray absorptiometry (DEXA) scans performed at baseline and 18 months post-therapy initiation. The primary study objective was to evaluate overall changes in lean and fat body masses between baseline and 18 months after therapy. The secondary objective was to evaluate regional changes in lean and fat body mass.
Between February 2018 and June 2021, 126 mHSPC patients were randomized to the two study arms. Of these 126, 87 patients were eligible for analysis (enzalutamide arm: 44, enzalutamide + zoledronic acid: 43). The baseline patient characteristics were well-balanced between the two arms, with the exception of a higher prevalence of ECOG performance status ≥1 in the enzalutamide + zoledronic acid arm.
After 18 months of therapy, fat body mass increased by 22.8% (p<0.001), while lean body mass decreased by 6.7% (p<0.001). The appendicular mass index decreased by 9.2% (p<0.001). There was no statistical difference in body composition changes between the two arms. None of the patients met the criteria for sarcopenic obesity; however, after 18 months of treatment, 11.8% of patients had a % fat body mass >40.8% and 3.5% had an appendicular mass index <5.5%.
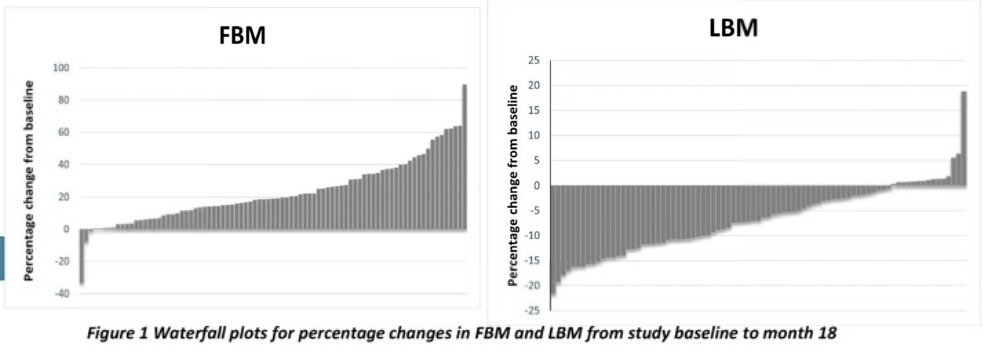
Younger patients (<70 years old) were more likely to experience an increase in fat body mass (p=0.017), but there were no age differences for loss of lean body mass (p=0.54).
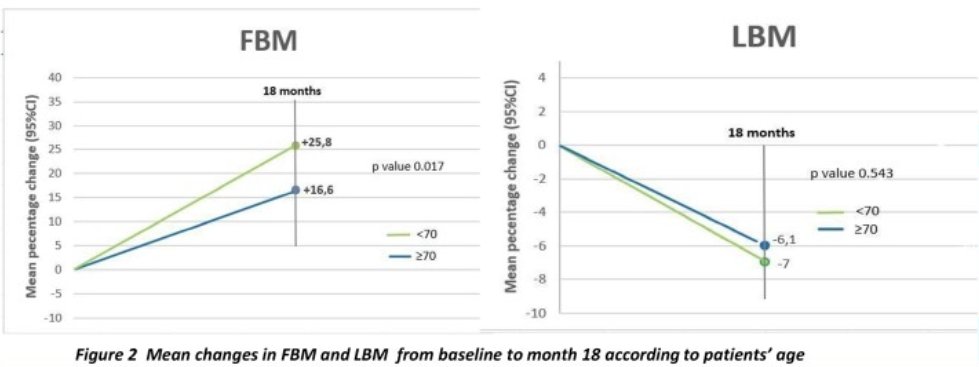
For regional body composition changes, fat body mass increases vary by body region. The highest increases were in the right arm (36.1%), and the lowest were in the head (3.7%). Lean body mass decrease varied by body region as well, with the largest loss in the right arm (-9.4%) left arm (-8.1%), and lower limbs (-8.1% and -5.8%).
Dr. Buffoni concluded as follows:
- This study showed a meaningful decrease in lean body mass and an increase in fat body mass in mHSPC patients treated with ADT and enzalutamide after 18 months of therapy, putting these patients at high risk of sarcopenic obesity
- Body composition changes were heterogeneous and differed by body region
- Fat body mass increase was more significant in patients <70 years of age
Presented by: Martina Buffoni, MD, Resident Physician, Medical Oncology, Spedali Civili di Brescia, Brescia, Italy
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


