(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a genitourinary cancers poster session. Dr. Lisa Horvath presented the results of an ad hoc analysis of the ENZAMET trial (ANZUP 1304) evaluating the association between the lipid biomarker, PCPro, and clinical outcomes in metastatic hormone-sensitive prostate cancer (mHSPC) patients.
In the ENZAMET trial, the addition of enzalutamide to standard of care androgen deprivation therapy (ADT) +/- docetaxel therapy was shown to improve overall survival in mHSPC patients, compared to ADT + non-steroidal antiandrogen (NSAA) +/- docetaxel 1,2 However, treatment resistance remains a problem, with 11% of mHSPC patients receiving enzalutamide + ADT +/- docetaxel experiencing mortality within two years of therapy.
PCPro is an assay of 3 ceramides, cholesterol, and triglycerides, and is a prognostic plasma lipid biomarker that was validated for mCRPC patients. Patients who are PCPro-positive were shown to have worse overall and radiographic progression-free survivals.3

The objective of this study was to evaluate whether PCPro is prognostic and/or predictive of enzalutamide responses in mHSPC patients, using a post-hoc analysis of the ENZAMET trial. PCPro status was available from 866 ENZAMET participants (ADT + enzalutamide [n=429]; ADT + NSAA [n=437]).
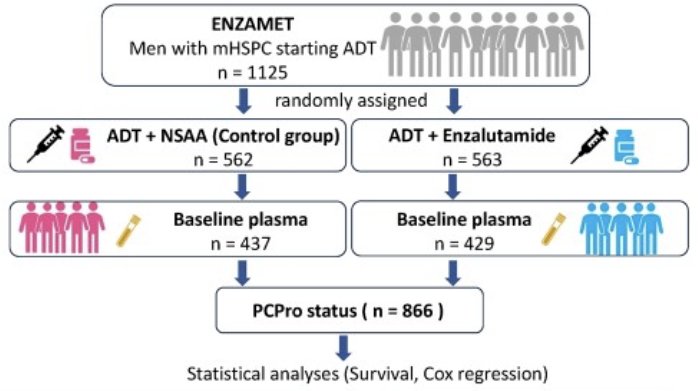
As previously described, the baseline patient characteristics by treatment arm were well-balanced.
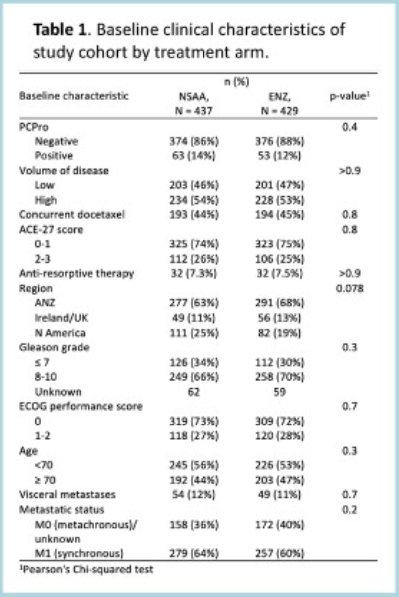
Of the 866 participants, 116 (13.3%) were PCPro-positive. PCPRO-positive patients were more likely to have high-volume disease (62% versus 52%, p=0.043), visceral metastases (18% versus 11%, p=0.026), and synchronous disease (70% versus 61%, p=0.059).
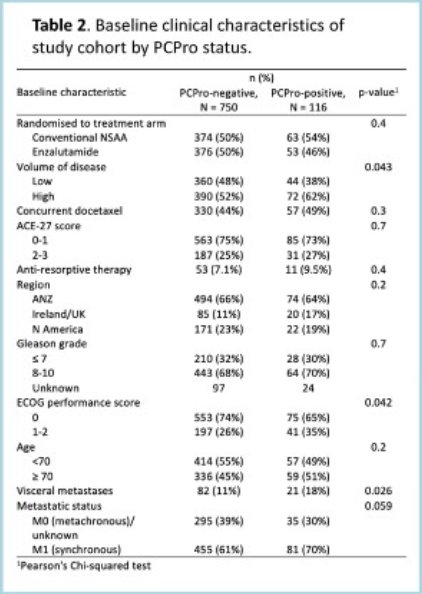
In the ENZAMET trial, PCPro-positive patients had worse overall survival (median: 50 months versus not estimable; 5-year overall survival: 63% versus 41%; hazard ratio [HR]: 1.81, p<0.001) and clinical progression-free survival (median: 44 versus 26 months; 5-year: 43% versus 22%; HR: 1.65, p<0.001).
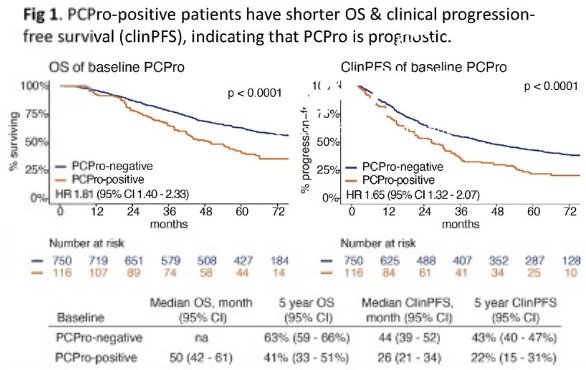
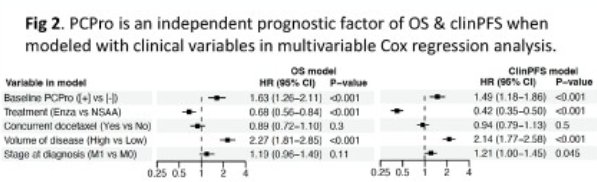
PCPro remained significantly prognostic of overall and clinical progression-free survivals on multivariable Cox regression analysis, adjusted for treatment arm, concurrent docetaxel use, volume of disease, and stage at diagnosis.
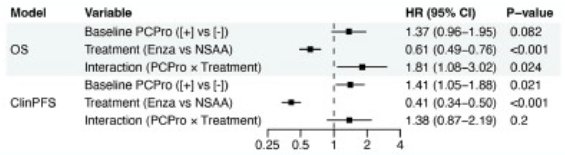
There was a significant interaction between PCPro status and treatment in in Cox regression analysis of overall survival, but not for clinical progression-free survival, suggesting that the OS benefit of enzalutamide differs by PCPro status.
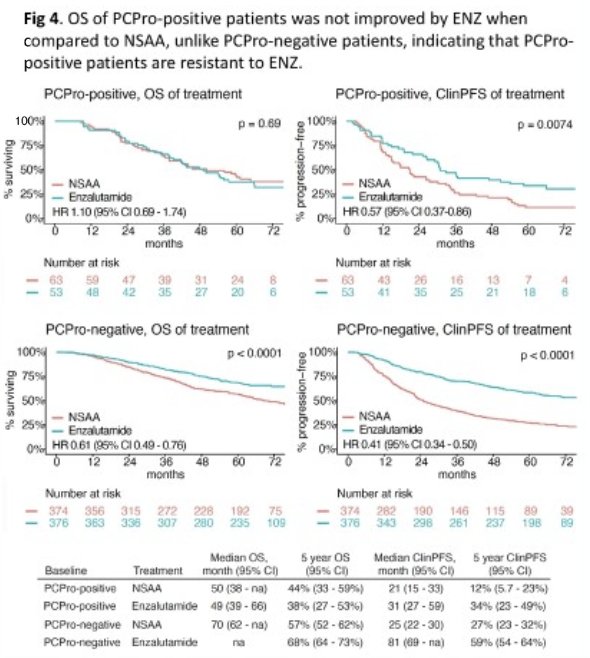
The overall survival of PCPro-positive patients was not improved by enzalutamide treatment, when compared to NSAA (median: 50 versus 49 months; HR: 1.10, p=0.69), unlike PCPro-negative patients (median: Not estimable versus 70 months; HR:0.61, p<0.0001), indicating that PCPro-positive patients may be resistant to enzalutamide therapy. However, PCPro-positive patients receiving enzalutamide had improved clinical progression-free survival, compared to NSAAs (median: 31 versus 21 months; HR: 0.57, p=0.0074).
Dr. Horvath concluded that:
- PCPro-positive patients have worse survival outcomes, compared to PCPro-negative patients
- Despite enzalutamide therapy improving clinical progression-free survival, PCPro-negative patients treated with enzalutamide have no overall survival benefit compared to those treated with non-steroid anti-androgens
Presented by: Lisa Horvath, MBBS, PhD, Director of the Department of Medical Oncology at Chris O’Brien Lifehouse, Syndey, New South Wales, Australia
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019; 381(2):121-31.
- Sweeney CJ, Martin AJ, Stockler MR, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): An international, open-label, randomized, phase 3 trial. Lancet Oncol. 2023; 24(4):323-34.
- Schienberg T, Lin HM, Fitzpatrick M, et al. PCPro: a clinically accessible, circulating lipid biomarker signature for poor-prognosis metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2024; 27(1):136-43.


