(UroToday.com) The 2024 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Kambiz Rahbar discussing the final safety and efficacy analysis of RaLu assessing 177Lu-PSMA therapy in patients with prior radium‑223. Radium-223 is an established alpha particle emitting radionuclide therapy with an acceptable safety profile. It prolongs overall survival and improves quality of life in patients with bone predominant metastatic castration-resistant prostate cancer (mCRPC).1 PSMA-targeted radioligand therapies have demonstrated anticancer activity [2]. More recently, 177Lu-PSMA was shown to have an acceptable safety profile and to extend overall survival in heavily pre-treated PSMA-positive patients with mCRPC, including taxane based chemotherapy and radium-223. The safety and efficacy of sequential use of radium‑223 and 177Lu-PSMA in patients with mCRPC were assessed in the real-world RaLu study [3] conducted across multiple centers in Germany and Israel.
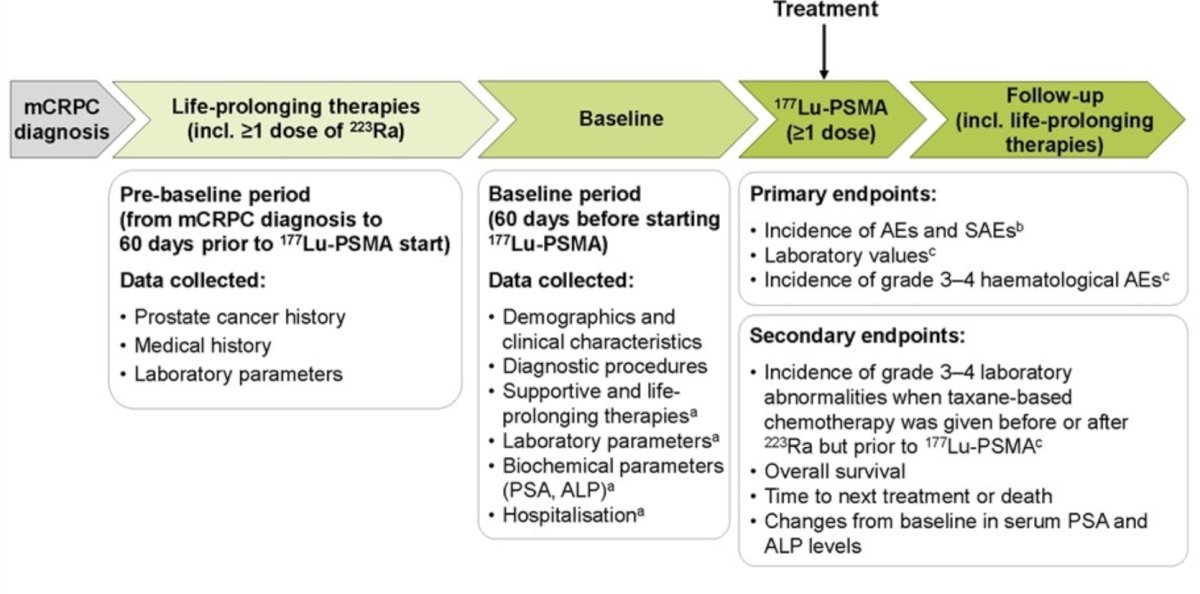
RaLu is a retrospective medical chart review of patients treated with radium-223 prior to 177Lu-PSMA. For this study presented at ESMO 2024, Dr. Rahbar and colleagues collected data from multiple center in Germany (2021–2022) and Israel (2022–2023) pertaining to patients with mCRPC who had received ≥1 radium‑223 dose and, in any subsequent therapy line, ≥1 177Lu-PSMA dose. The study schema is as follows:
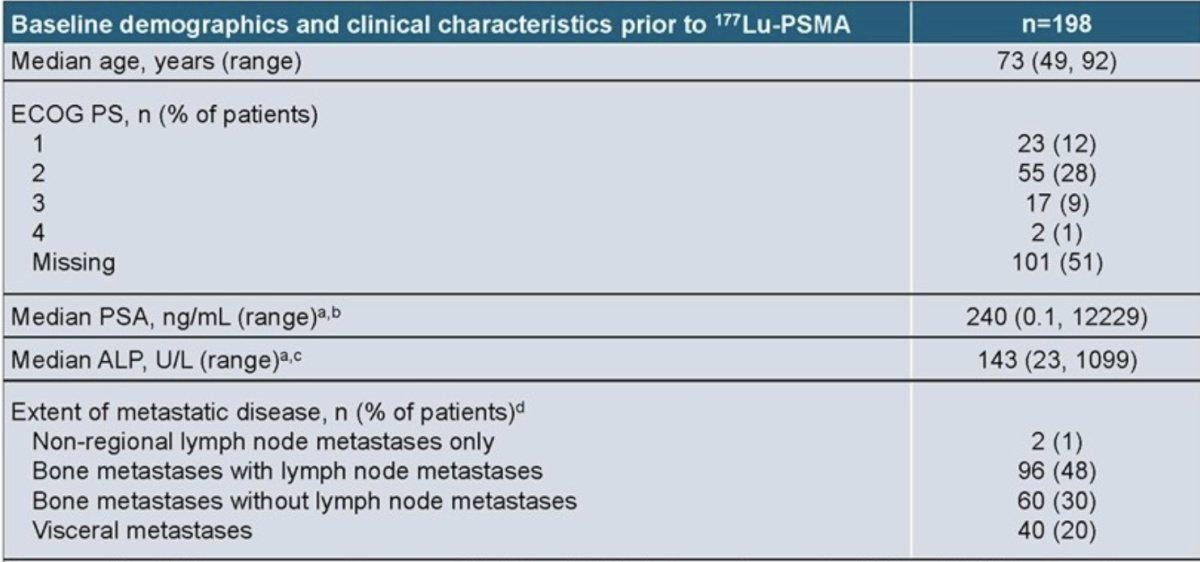
Overall, data from 198 patients were analyzed. At the start of 177Lu-PSMA therapy, the median age was 73 years and 20% of patients had visceral disease. The median PSA level was 240 ng/ml (range: 0.1 – 12,229) and median ALP level was 143 U/L (range: 23-1,099):
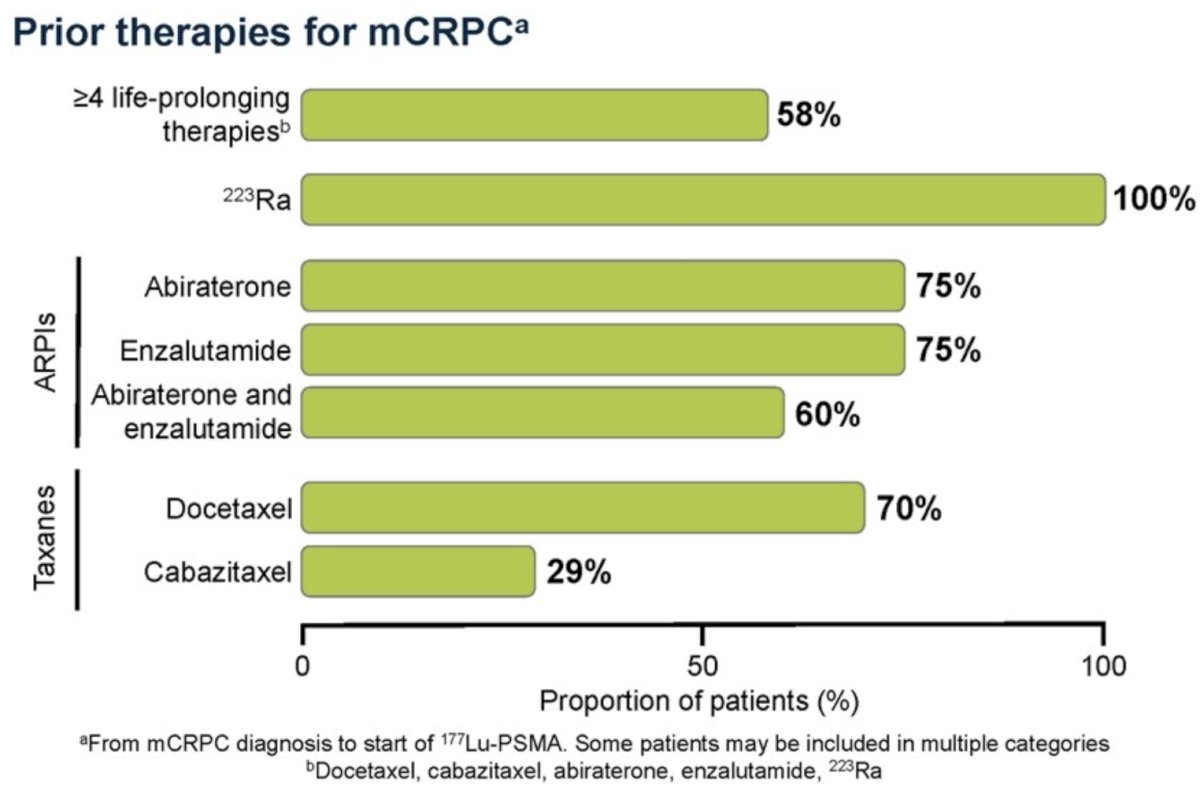
Overall, 58% of patients had received ≥4 life-prolonging therapies before starting 177Lu-PSMA. All patients had received radium‑223 (66% had received six radium‑223 injections). Other prior treatments included abiraterone (75%), enzalutamide (75%), docetaxel (70%) and cabazitaxel (29%):
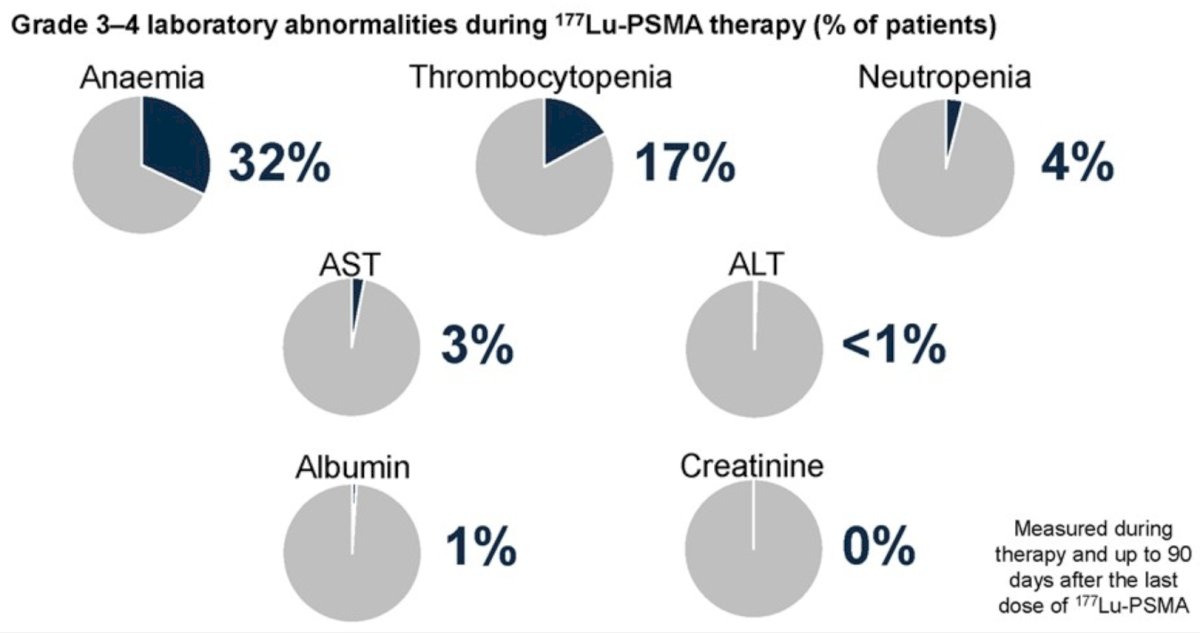
There were 38% of patients that received ≥4 177Lu-PSMA cycles, with a median completion of 3 cycles. The most common grade 3-4 adverse events included anemia (32%) and thrombocytopenia (17%):
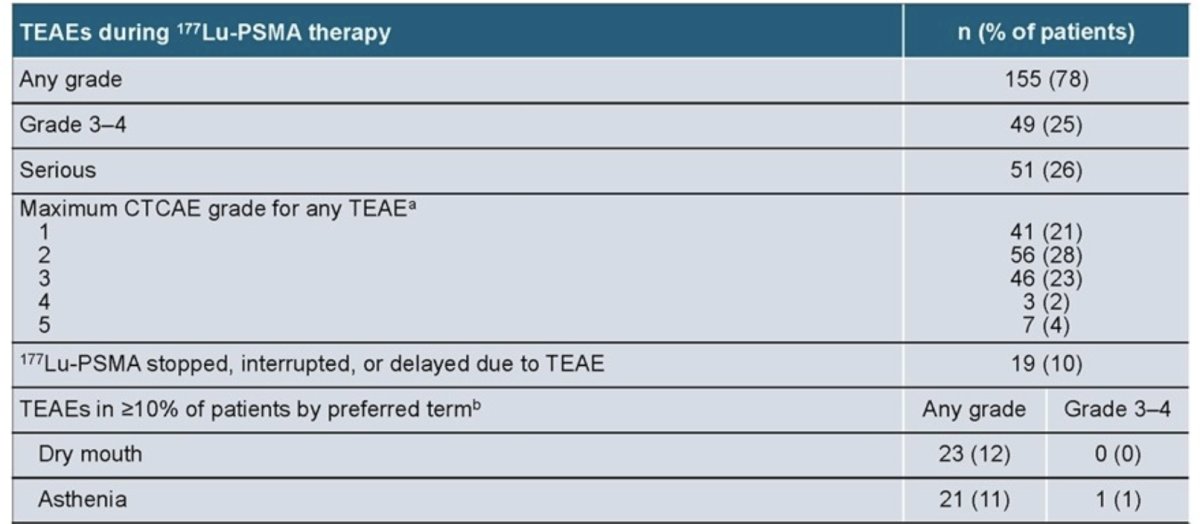
Treatment-emergent adverse events with 177Lu-PSMA were mainly hematological and non-fatal. Excluding laboratory abnormalities, the most common (≥10% of patients) any-grade treatment-emergent adverse events were dry mouth (12%) and asthenia (11%):
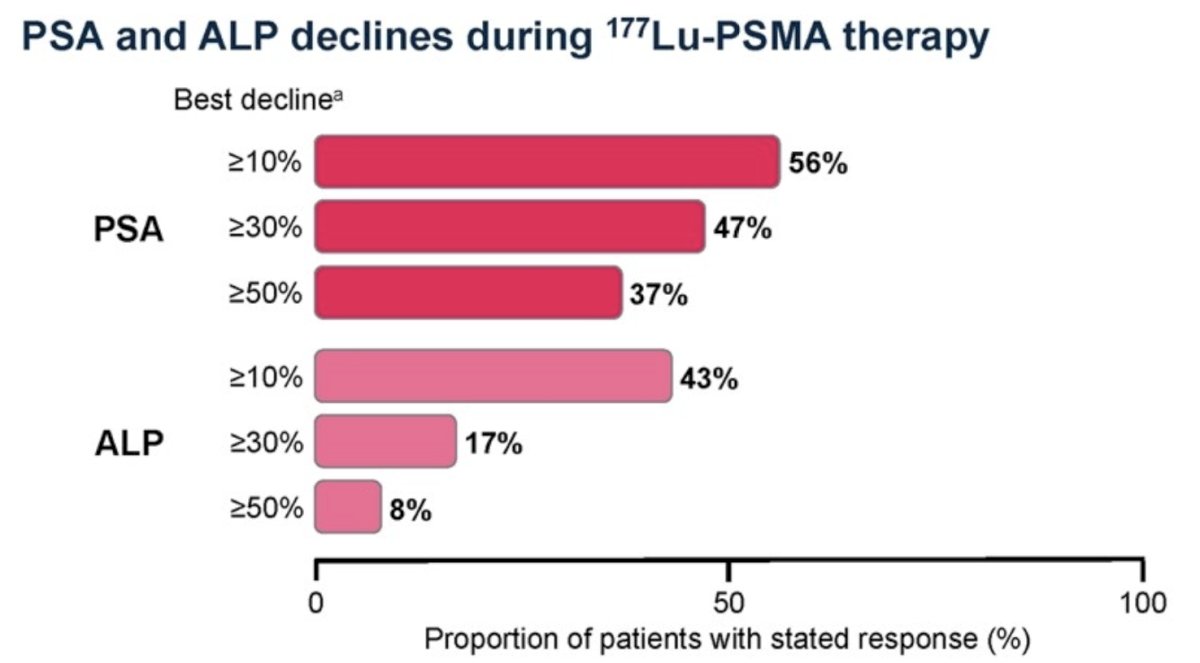
PSA data were available for 102 patients during 177Lu-PSMA treatment: a PSA decline of ≥30% or ≥50% occurred in 48 (47%) and 38 (37%) patients, respectively. Corresponding ALP declines occurred in 13 (17%) and 6 (8%) patients (among 77 patients):
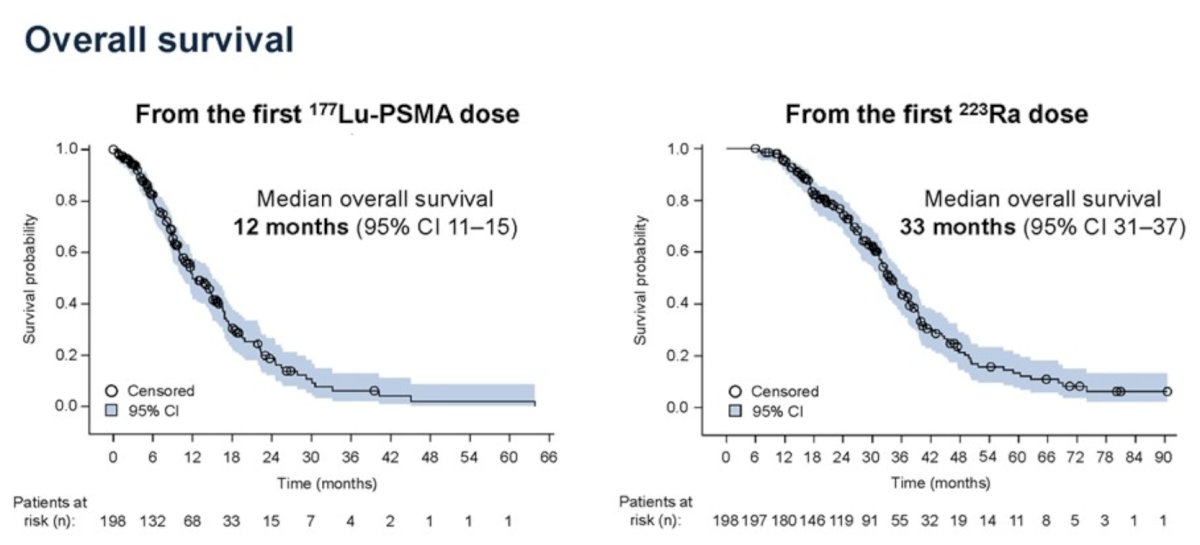
Median overall survival was 12.0 months (95% CI, 10.5–15.1) from the start of 177Lu-PSMA and 33.4 months (95% CI, 31.4–37.3) from the start of radium‑223:
Dr. Rahbar concluded his presentation discussing the final safety and efficacy analysis of RaLu assessing 177Lu-PSMA therapy in patients with prior radium‑223 with the following take-home points:
- These real-world findings support the clinical feasibility of treating patients with mCRPC with 177Lu-PSMA following treatment with radium‑223
- These insights may provide valuable guidance for optimizing treatment sequencing and decision-making in clinical practice
Presented by: Kambiz Rahbar, MD, University Hospital Muenster, Muenster, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Rahbar K, Essler M, Pabst KM, et al. Safety and Survival Outcomes of 177Lu-Prostate-Specific Membrane Antigen Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer with Prior 223Ra Treatment: The RALU Study. J Nucl Med. 2023 Apr;64(4):574-578.


