(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a genitourinary cancers poster session. Dr. Evan Yu presented the results of ARROW, a multicenter randomized phase II trial of PSMA-targeted radioligand therapy with 131I-LNTH-1095 plus enzalutamide versus enzalutamide alone in chemotherapy-naïve metastatic castrate-resistant prostate cancer (mCRPC) patients with disease progression on abiraterone and positive piflufolastat F 18 scans.
Pre-clinical studies have demonstrated that PSMA-targeted radioligand therapy plus enzalutamide, an androgen receptor pathway inhibitor, have synergistic mechanisms of action.
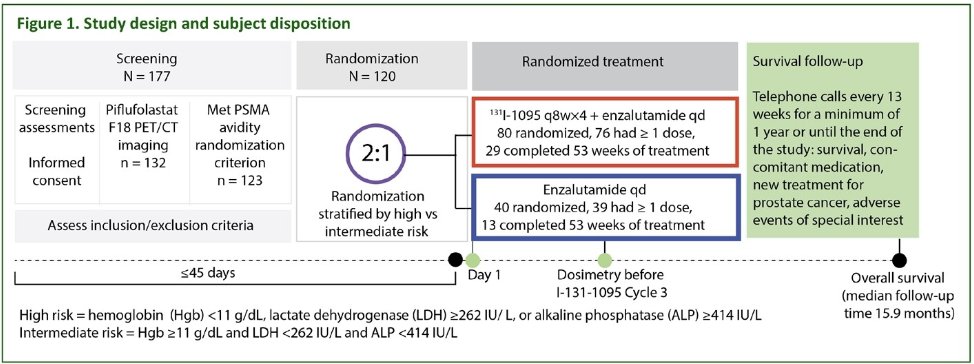
ARROW (NCT03939689) recruited men (≥18 years) who were planned for enzalutamide following mCRPC disease progression on abiraterone therapy and who were ineligible for or refused taxane-based chemotherapy. The initial prostate cancer diagnosis was confirmed by histopathology and imaging per RECIST 1.1 following the Prostate Cancer Working Group 3 (PCWG3) criteria. PSMA avidity was confirmed using Piflufolastat F 18. Eligible subjects were randomized 2:1 either to enzalutamide (160 mg orally once daily) plus 1311-LNTH-1095 (100 mCi dose, then up to 4 cycles ≥8 weeks apart, as determined by dosimetry and dose-limiting events) or to enzalutamide monotherapy.
The primary study endpoint was PSA50 (i.e., percentage of subjects with ≥50% decrease from baseline in serum prostate-specific antigen [PSA], confirmed ≥3 weeks later). The secondary efficacy endpoints were:
- Objective response rate
- Radiographic progression-free survival
- Overall survival
- Time to PSA progression (PCWG3 criteria)
- Duration of response
- Time to next treatment for prostate cancer
- Event-free rate.
- Safety: Treatment-emergent adverse events, graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE v.5).
The sample size calculations are summarized below:

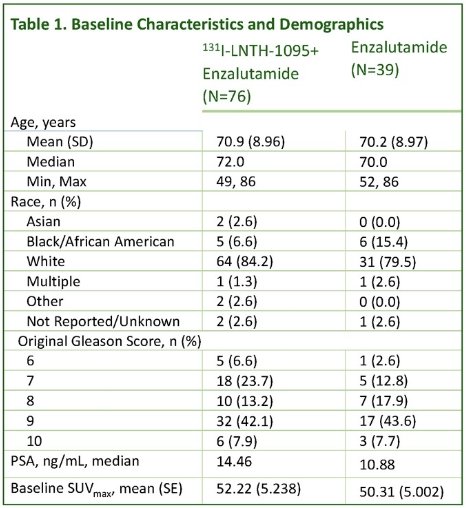
The baseline patient characteristics are summarized in the table below. The median patient age was 70–72 years. The majority of patients (≥80%) were White. The median baseline PSA was 14.5 in the experimental arm and 10.9 in the control arm. The mean baseline SUVmax was 52.2 and 50.3 in the experimental and control arms, respectively.
The most common reasons for discontinuation of 131I-LNTH-1095 + enzalutamide, as compared to enzalutamide monotherapy were:
- Radiographic progression: 21% vs 35%
- Lack of treatment benefit: 7.5% vs 12.5%
- Treatment-emergent adverse event: 10% vs 2.5%
- Experimental arm: thrombocytopenia (n=3), febrile neutropenia (n=1), pancytopenia (n=1)
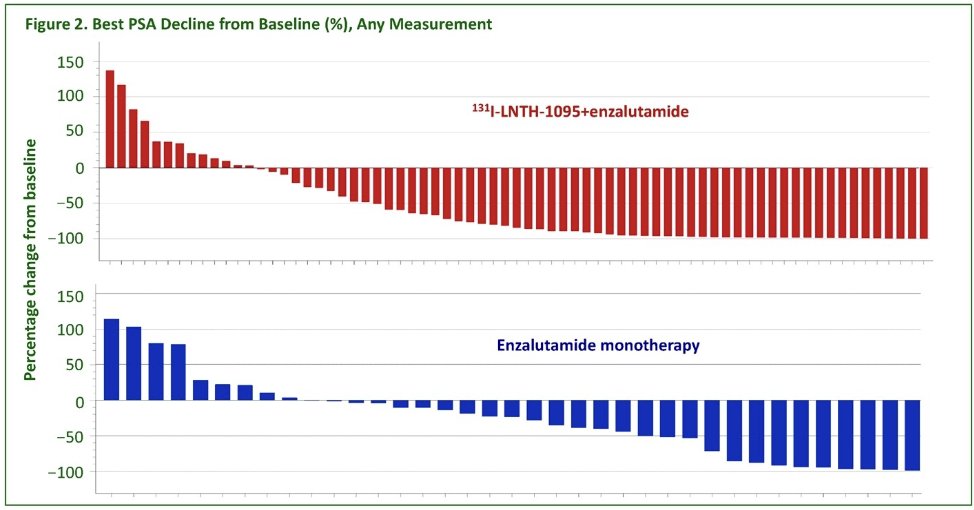
From an efficacy standpoint, a PSA50 response was observed in 63% of patients in the 131I-LNTH-1095 + enzalutamide arm versus 31% of patients in the enzalutamide monotherapy arm (p=0.003). Subgroup analyses for age, race, ethnicity, and baseline LDH demonstrated a generally consistent effect. Summarized in the figure below is the best PSA decline from baseline by treatment group.
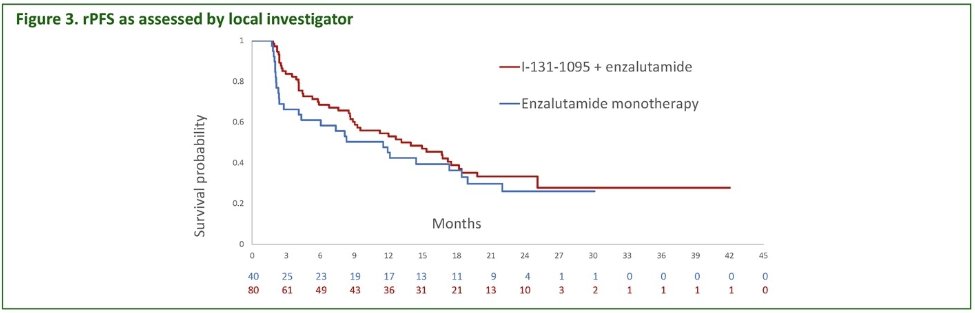
The median radiographic progression-free survival favored the 131I-LNTH-1095 + enzalutamide arm (14 versus 11.5 months, p=0.10). However, the study was not adequately powered to detect statistically significant improvements for this outcome.
Over a median study follow-up duration of 15.9 months (data cutoff: September 21, 2023), the median overall survival was 18.8 months (95% CI: 15.3–29.5) for 131I-LNTH-1095 + enzalutamide versus 22 months (95% CI: 14.6 – not estimable) for enzalutamide monotherapy (p=0.59). At this study cut-off, <50% of events had occurred in both treatment arms (40% for 1311-LNTH-1095 + enzalutamide; 34% for enzalutamide monotherapy).
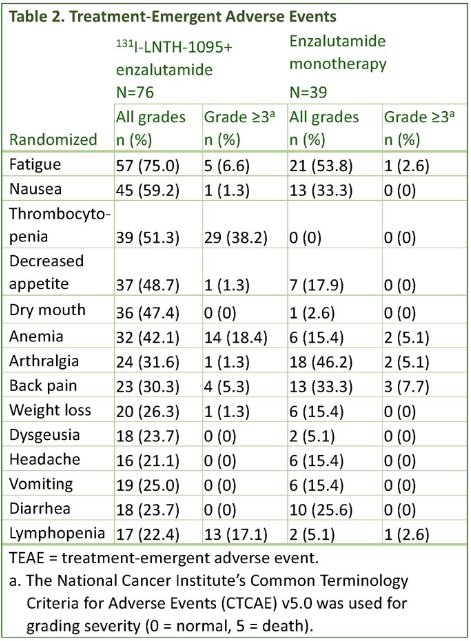
Nearly all subjects (96% for 1311-LNTH-1095 + enzalutamide and 95% for enzalutamide monotherapy) had ≥1 treatment-emergent adverse event. The incidence of grade ≥3 treatment-emergent adverse events were 66% and 41% for the experimental and control arms, respectively. Treatment-emergent adverse events with an incidence ≥20% are summarized below:
Dr. Yu concluded as follows:
- ARROW met its primary efficacy endpoint: a statistically significant higher PSA50 (63% versus 31%, p=0.003) among subjects who received 131I-LNTH-1095 in addition to standard-of-care enzalutamide.
- The safety results show that 131I-LNTH-1095 has a manageable safety profile consistent with that of other agents in this class.
- These promising efficacy results may serve as a foundation for a potential next-generation product candidate with an alternative radioisotope.
Presented by: Evan Yu, MD, Professor, Department of Medicine, University of Washington and Fred Hutchinson Cancer Research Center, Seattle, WA
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: ARROW Study Reveals Iodine-131 PSMA Efficacy in Advanced Cancer - Evan Yu


