(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a genitourinary cancers poster session. Dr. Jason Redman presented the results of the Quick Efficacy Seeking Trial (QuEST1) trial of combination BN-brachyury (BNVax) + bintrafusp alfa + nogapendekin alfa inbakicept-pmln (N-803) in castrate-resistant prostate cancer.
Although novel treatment options for castrate-resistant prostate cancer (CRPC) patients have emerged over the last few years, and numerous single-agent and combination therapies have been approved for use in clinical practice, such patients still have poor prognoses with estimated median overall survivals of two to three years.
To date, trials of immune checkpoint inhibitors in the advanced prostate cancer setting have failed to demonstrate clinically meaningful survival benefits. Immune checkpoint blockade (ICB) currently has no treatment role in unselected prostate cancer patients, with several studies having failed to demonstrate reproducible clinical benefit for ICB monotherapy. However, there is evidence that such therapy may be efficacious in patients with microsatellite instability high (MSI-H)/mismatch repair deficient (dMMR) and/or high tumor mutational burden (≥10 mutations per megabase) tumors.1,2 Since patients with MSI-H/dMMR or high tumor mutational burden tumors represent a small proportion of prostate cancer patients (~5%),3 there is interest in investigating combination immunotherapies for CRPC by selecting agents from a growing developmental pipeline in order to exploit the potential synergies of multiple therapies.
Given evidence that therapeutic vaccines can generate tumor-infiltrating immune responses to prostate cancer,4,5 Dr. Redman and colleagues sought to develop a combination regimen aimed at enhancing the anti-tumor activity of a poxviral vaccine (BN-brachyury) targeted to the transcription factor, brachyur. Brachyury is key to carcinoma cells’ ability to invade and metastasize6 and is associated with poor prognostic features in prostate cancer.7
Dr. Redman and colleagues selected therapeutic combinations that could have synergistic mechanisms of action to enable anti-tumor immune responses in an adaptive study design of four drug agents. Preliminary results of this study (QuEST1) have been previously published.8 In this report, the study investigators presented the final results from the three-drug arm combining BN-brachyury vaccine with an anti-PD-L1 monoclonal antibody fused to the TGF-B-RII receptor extracellular domain (bintrafusp alfa [BA]) and an IL-15 superagonist complex (nogapendekin alfa inbakicept-pmln [N-803]) that is FDA-approved for BCG-unresponsive non-muscle invasive bladder cancer.![final results from the three-drug arm combining BN-brachyury vaccine with an anti-PD-L1 monoclonal antibody fused to the TGF-B-RII receptor extracellular domain (bintrafusp alfa [BA]) and an IL-15 superagonist complex (nogapendekin alfa inbakicept-pmln [N-803]) that is FDA-approved for BCG-unresponsive non-muscle invasive bladder cancer](/images/com-doc-importer/173-esmo-2024/esmo-2024-results-from-the-quick-efficacy-seeking-trial-quest1-arm-combining-bn-brachyury-bnvax-bintrafusp-alfa-nogapendekin-alfa-inbakicept-pmln-n-803-in-castration-resistant-prostate-cancer/image-0.jpg)
This was a single-center study conducted at the National Institutes of Health Clinical Center, Bethesda, MD, USA (NCT03493945). Patients with either non-metastatic (nmCRPC) or metastatic CRPC (mCRPC) who had a rising PSA (>1 ng/mL) and did not require narcotics regularly for prostate cancer-related pain were enrolled into Arms 2.2A and 2.2B, per the trial design below:
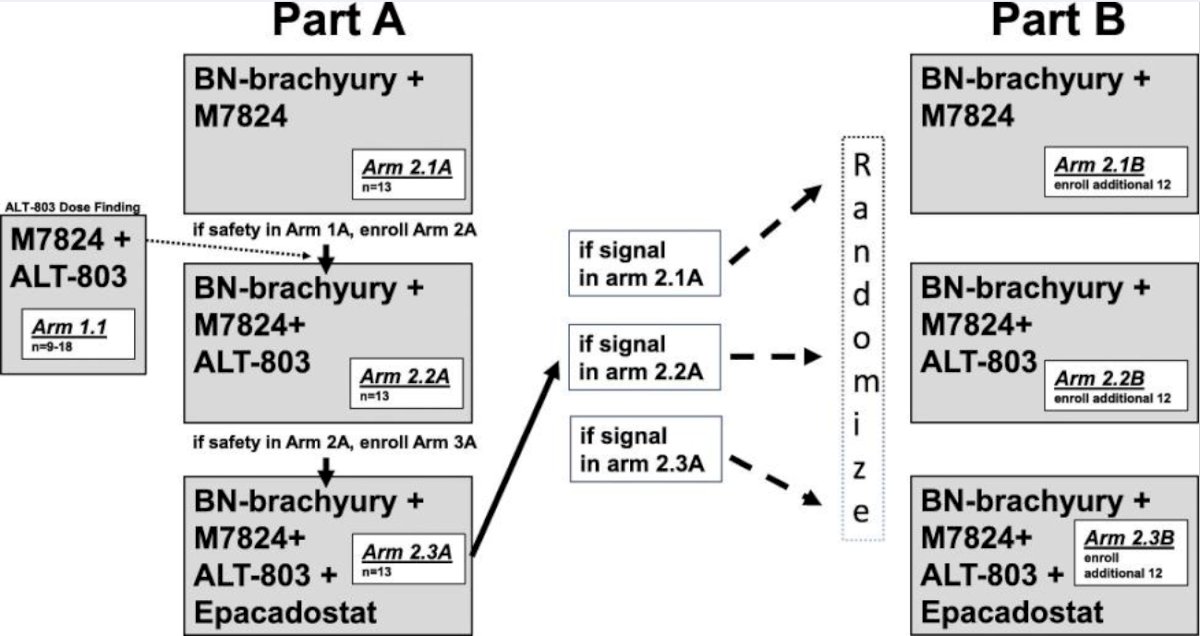
Per study protocol (Simon two-stage design), enrollment to Arm 2.2B (n=12) began if Arm 2.2A (n=13) arm had ≥2 clinical responses, as defined below. A protocol amendment approved in 2021 allowed individuals progressing on an androgen receptor pathway inhibitor (ARPI) to continue ARPI on study.
The BN-Brachyury vaccine platform was administered as a priming dose (MVA-BN-brachyury 2.0 x 108 IU subcutaneously) on day 1 of both cycles 1 and 2. This was followed two weeks later by booster doses (FPV-Brachyury 1.0 x 109 IU subcutaneously) started two weeks after the second dose of MVA-BN-Brachyury, every four weeks until six months, and finally every three months until reaching two years from the first dose of MVA-BN-Brachyury. Bintrafusp alfa 1,200 mg IV was administered on days 1 and 15 of each 28-day cycle. N-803 15 µg/kg subcutaneously was administered on days 1 and 15 of each 28-day cycle. De-escalation of N-803 dosing to 10 or 8 µg/kg was allowed for N-803 attributed toxicities, per investigator's discretion.
The primary objective was to determine the clinical benefit (i.e. response rate) for this regimen. Response was defined as partial or complete responses by RECISTv1.1 if RECISTv1.1-measurable disease was present at baseline, or by a PSA decrease of ≥30% from baseline sustained for > 21 days. Patients underwent CT imaging and technetium bone scan at baseline and every three months thereafter. Secondary endpoints included progression-free survival and safety outcomes, assessed using CTACAE version 5.0.
The secondary endpoints were progression-free survival and safety outcomes. Adverse events were graded using the CTCAE version 5.0.
At the study cut-off date of April 2024, 25 patients had been enrolled and followed for a 0.25–54 months. The median patient age was 66 years, and the median PSA was 17.6 ng/dL. The baseline patient characteristics, including prior treatments received, are summarized in the Table below.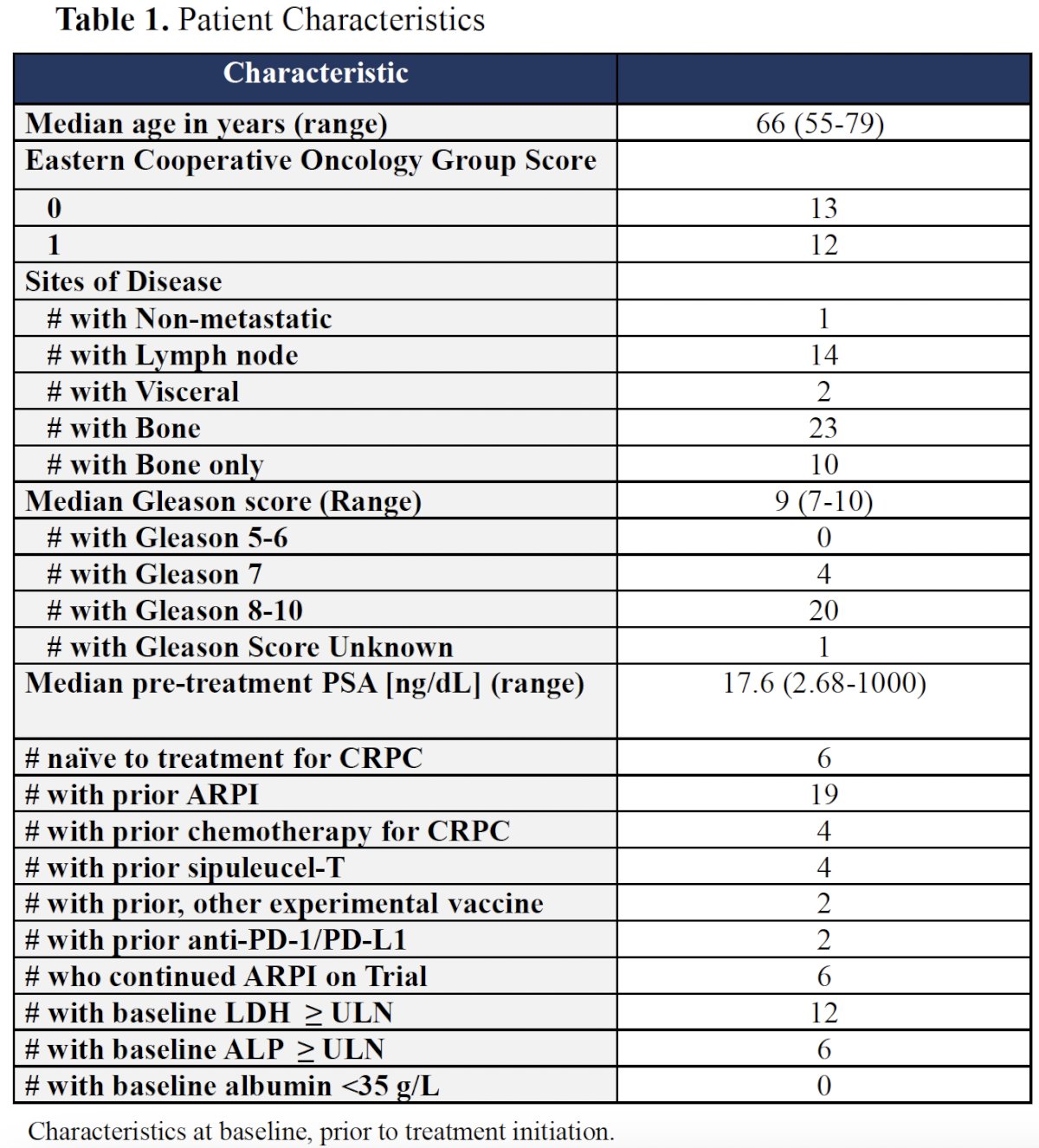
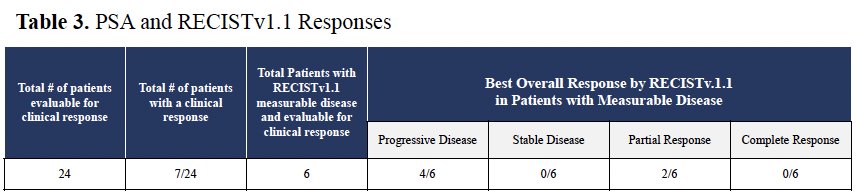
Seven of 24 patients (29%) evaluable for response had PSA responses (1 had dMMR). Of the 7 responders, two patients, who were mismatch repair proficient, had confirmed partial responses. There were no complete responses, and none had evidence of stable disease. One of the responding individuals had a germline CDK12 mutation and had not previously received immune checkpoint blockade.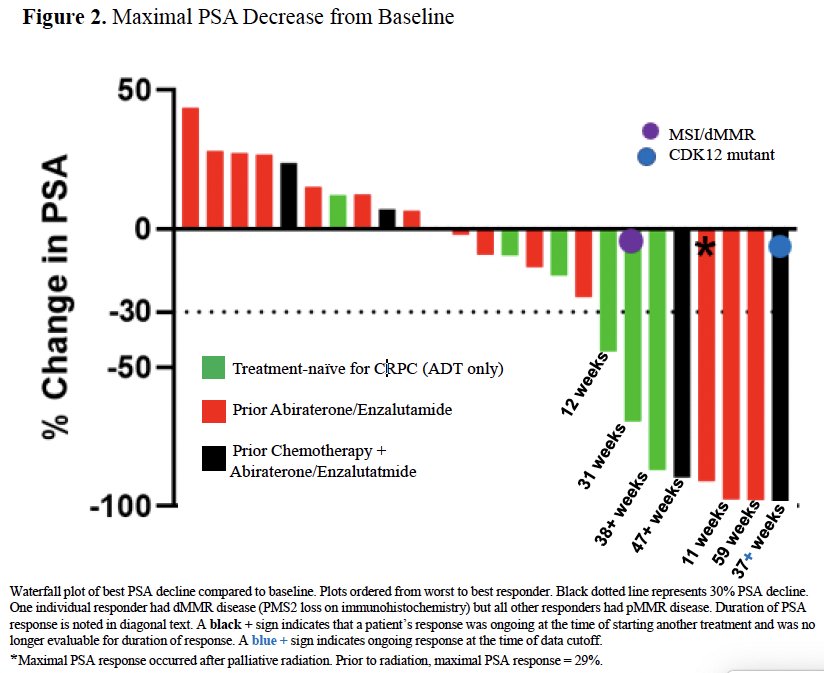
The 6-month progression-free survival was 35% (95% CI: 16–54%), and the 36 months overall survival was 67% (95% CI: 40–84%).
Treatment-related adverse events (TRAEs) are summarized in the table below. Five patients had grade 3 TRAEs: hematuria, non-infective cystitis, anemia, and eosinophilia. One patient developed diabetes mellitus (grade 3) medically managed with insulin replacement. The same individual also developed grade 4 neutropenia that resolved after a steroid taper. Three patients (all PSA responders) developed central adrenal insufficiency manageable with physiologic hormone replacement. There were no grade 5 events.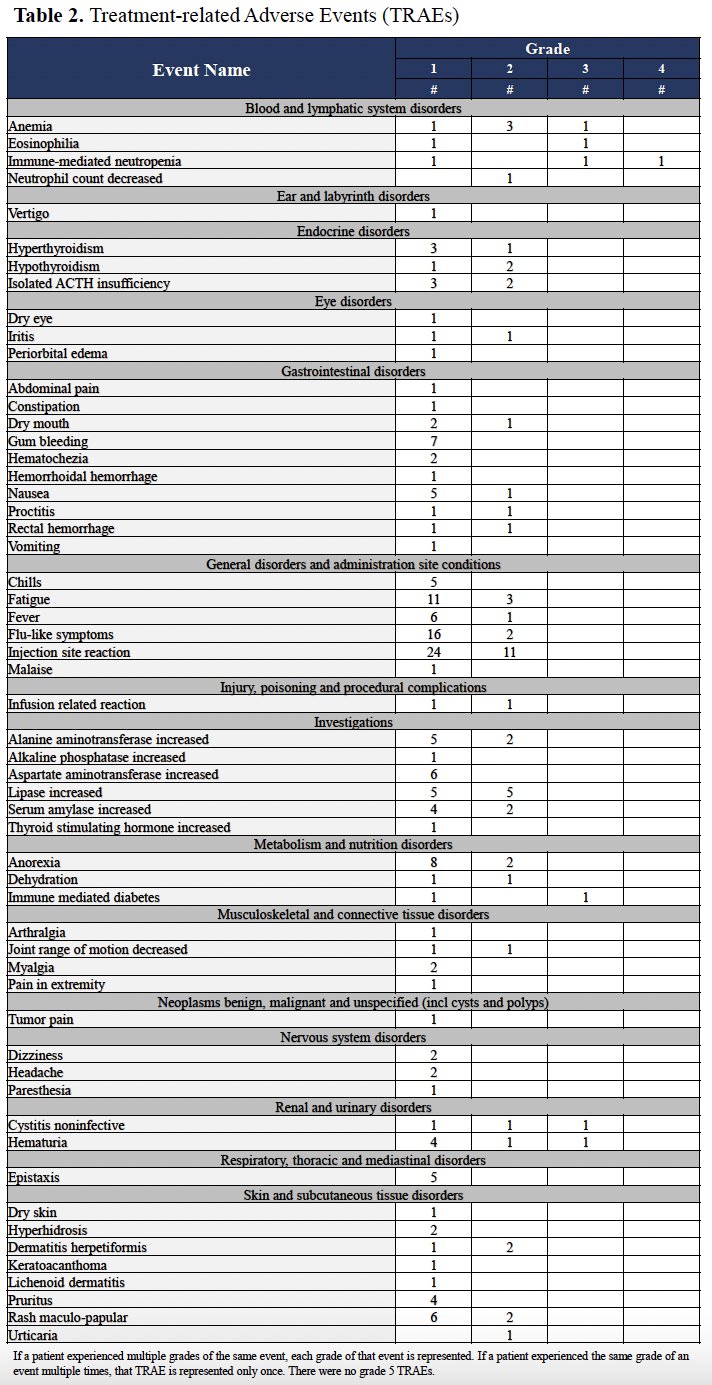
Dr. Redman concluded as follows:
- The combination of BN-brachyury + bintrafusp alfa + N-803 shows activity in this heterogenous group of CRPC patients, including 6 individuals with mismatch repair proficient disease
- This provides proof of concept that combination immunotherapy regimens that provide immune enhancements beyond enabling the immune response (i.e., also engage and expand) can have anti-tumor activity in CRPC.
- In general, the toxicity profile was consistent with the known profile of each individual agent.
- As expected, immune-related toxicities occurred; however, an unexpectedly high incidence of adrenal insufficiency was observed and only occurred in responders
- Additional studies are needed to understand the etiology of this presumably immune-mediated central adrenal insufficiency and its potential contribution to anti-tumor activity.
Presented by: Jason M. Redman, MD, Assistant Research Physician, National Cancer Institute, Bethesda, MD
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015; 372(26):2509-20.
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017; 357(6349):409-13.
- Abida W, Cheng ML, Armenia J, et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019; 5(4):471-8.
- Sheikh Nam Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013; 62(1):137-47.
- Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014; 106(11):dju268.
- Fernando RI, Litzinger M, Trono P, et al. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010; 120(2):533-44.
- Pinto F, Pertega-Gomes N, Pereira MS, et al. T-box transcription factor brachyury is associated with prostate cancer progression and aggressiveness. Clin Cancer Res. 2014; 20(18):4949-61.
- Redman JM, Steinberg SM, Gulley JL. Quick efficacy seeking trial (QuEST1): a novel combination immunotherapy study designed for rapid clinical signal assessment metastatic castration-resistant prostate cancer. J Immunother Cancer. 2018; 6(1):91.


