(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a genitourinary cancers poster session. Dr. Vincent Habouzit presented the results of a nationwide study evaluating factors associated with clinical and biologic responses in French patients receiving [177Lu]Lu-PSMA-617 under France’s early access program.
177Lu-PSMA-617 is a radiopharmaceutical with binding affinity to the prostate specific membrane antigen (PSMA), expressed in 90% of metastatic castration resistant prostate cancer (mCRPC) patients.1 The VISION study showed that 177Lu-PSMA-617, combined with best standard of care, prolonged progression-free survival (PFS), overall survival, and delayed time to worsening of health-related quality of life in PSMA-positive mCRPC patients previously treated with ≥1 taxane-based chemotherapy and one androgen receptor pathway inhibitor (ARPI).2 A cohort temporary authorization for use has been granted to 177Lu-PSMA-617 by French Health Authorities for patients with this indication. This early access program began on December 1, 2021, and is still ongoing.
Various factors related to patients, their disease, and the treatment sequence may impact the treatment's effectiveness. This study is a retrospective analysis that aims to evaluate the influence of these factors on the clinical and biological response of patients receiving 177Lu-PSMA-617 under France’s early access program.
177Lu-PSMA-617 was given to patients with progressive mCRPC overexpressing PSMA, previously treated with ≥1 taxane chemotherapy and ≥1 ARPI. Patients included had received at least one of the six planned cycles of intravenous infusions of 177Lu-PSMA-617 (7.4 GBq +/- 10%) administered every six weeks.
In order to ensure a minimum of 6-month follow-up after the first injection, the efficacy data focused on patients included from December 1, 2021, to September 30, 2023. The patient’s baseline characteristics were described from the total patient population included in this early access program from December 1, 2021, to June 30, 2024.
Patients were categorized into two groups: Responders (experiencing reduced PSA levels and improved clinical symptoms) versus non-responders (experiencing PSA progression and/or worsening clinical symptoms). Response evaluation was conducted by the referring nuclear medicine physician during routine care follow-up visits. Group characteristics were compared using bivariate analysis. Additionally, the impact of factors associated with treatment response on progression-free survival (PFS) was evaluated.
From December 1, 2021, to June 30, 2024, 2,251 patients were included. Among them, 684 were classified as responders (30.4%) and 1,567 as non-responders (69.6%). Patient characteristics are summarized below:
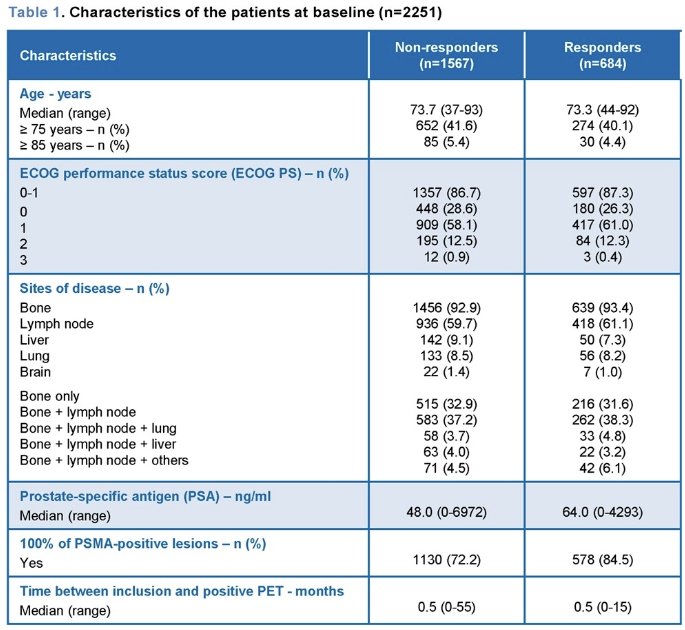
Efficacy data and statistical comparisons were assessed from 1,048 patients included until September 30, 2023, including 466 responders (44.5%) and 582 non-responders (55.5%).
Responders were less likely to have had brain metastases, but more likely to have had all their metastatic lesions be PSMA positive, received all planned cycles of 177Lu-PSMA-617, received concurrent treatment (ARPI or bisphosphonates), and ≥1 reduction in opioid analgesic treatment requirement during follow-up.
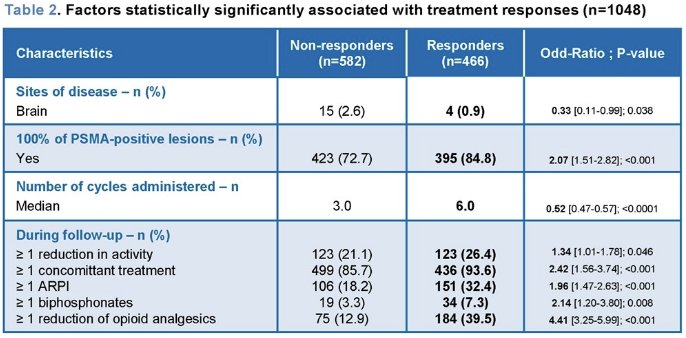
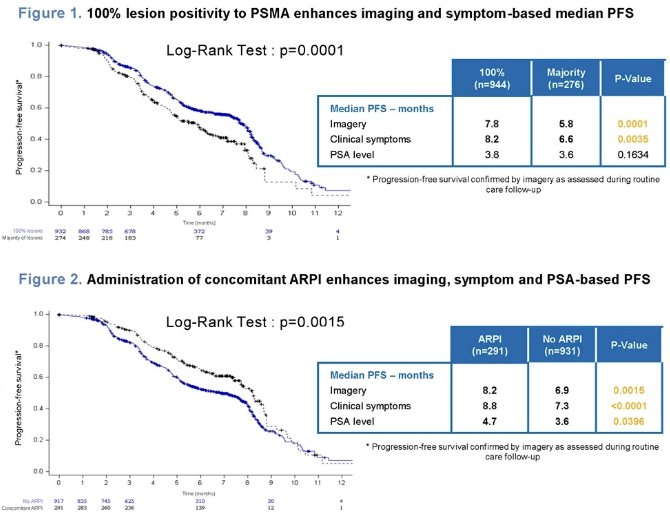
The study investigators further evaluated the impact of factors associated with clinical and biological treatment responses on PFS, as assessed by imaging, clinical examination, and PSA evaluation during routine care follow-up. Their results suggested that the presence of 100% PSMA lesion positivity and the use of concomitant treatment, particularly concomitant ARPI, contributed to improved PFS.
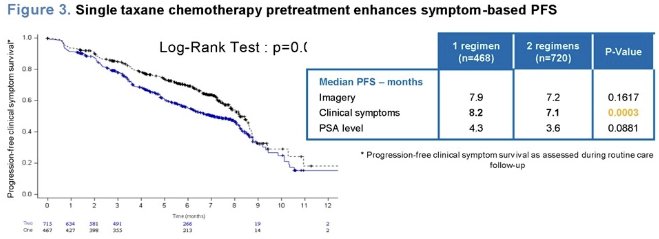
Additionally, they observed that patients who had received a single course of taxane chemotherapy had longer survival without symptom progression.
Dr. Habouzit concluded as follows:
- An early access program has been granted to 177Lu-PSMA-617 in France for patients with progressive mCRPC expressing PSMA, previously treated with ≥1 taxane chemotherapy and ≥1 ARPI.
- From December 1, 2021, to September 30, 2023, 1,048 PSMA-PET-positive mCRPC patients were included in this early access program. They were classified as responders (44.5%) and non-responders (55.5%) based on clinical and biological follow-up.
- The characteristics of the patients, their disease, and the therapeutic sequences recorded in the early access program database were compared between the two populations using a bivariate analysis. Patients classified as clinical/biological responders were more likely to:
- Have not developed brain metastases and have all their lesions be PSMA-positive.
- Have received concomitant treatment, in particular ARPI or bisphosphonates.
- Have had a reduction in opioid analgesic treatment during follow-up.
- Have received all six courses of treatment.
- Further analysis showed that PSMA-PET lesion positivity, concomitant treatment, particularly concurrent ARPI administration, also contribute to enhanced imaging and symptom-based progression-free survival.
Presented by: Vincent Habouzit, MD, MSc, Department of Nuclear Medicine, University Hospital of Saint-Etienne, Saint Etienne, France.
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Paschalis A, Sheehan B, Riisnaes R, et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur Urol. 2019;76(4):469-78.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021; 385(12):1091-103.


