(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the presentation of poster 1637. Dr. Neeraj Agarwal presented a post hoc analysis from both cohorts in TALAPRO-2 study, exploring the efficacy of talazoparib and enzalutamide in metastatic castration-resistant prostate cancer (mCRPC) patients previously treated with androgen receptor pathway inhibitors (ARPI) or docetaxel.
TALAPRO-2 (NCT03395197) is a Phase 3, randomized, double-blind, placebo-controlled trial evaluating talazoparib plus enzalutamide versus placebo plus enzalutamide in the first-line treatment setting for patients with metastatic castration-resistant prostate cancer (mCRPC). This study includes both unselected (all-comers) patients and those with alterations in homologous recombination repair (HRR) genes. Notably, prior to randomization, all patients were prospectively assessed for HRR gene alterations in tumor tissue and/or circulating tumor DNA (ctDNA) using FoundationOne CDx and/or FoundationOne Liquid CDx.1
Dr. Agarwal briefly summarized the results of TALAPRO-2: In the all-comers population, talazoparib plus enzalutamide was associated with a 37% reduction in the risk of radiological progression. In the HRR-deficient population (N=399), treatment with talazoparib plus enzalutamide was associated with a 55% reduction in the risk of radiological progression. Figure 1 below shows the number of patients in the all-comers and HRR-deficient population who received prior ARPI or docetaxel in the castration-sensitive setting.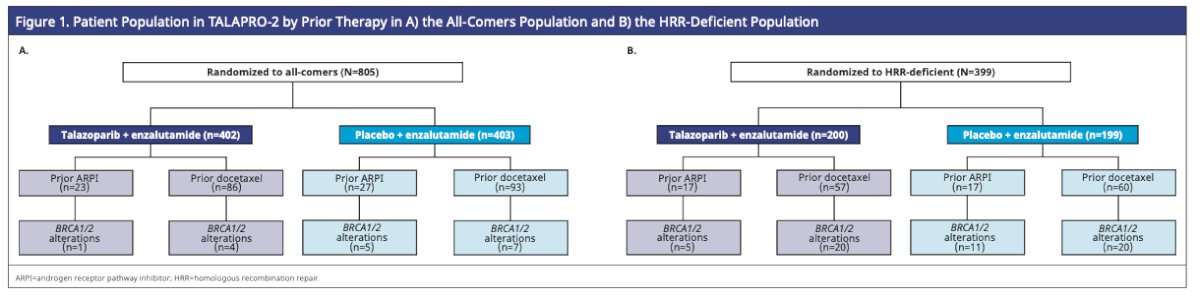
In the all-comers cohort, among patients who had been previously treated with an ARPI, 65% in the talazoparib plus enzalutamide group experienced a radiological progression-free survival (rPFS) event, compared to 59% in the placebo plus enzalutamide group. The median rPFS was 11.0 months in the talazoparib plus enzalutamide arm versus 1.9 months in the placebo plus enzalutamide arm (HR=0.57; 95% CI, 0.28–1.16; P=0.12) for patients previously treated with an ARPI.
Importantly, Dr Agarwal acknowledges that the small number of patients who were previously treated with an ARPI (n=50) In the all-comers population and the asymmetric distribution of the data may have confounded these results.
In patients previously treated with docetaxel in the hormone-sensitive setting within the all-comers population, 34% experienced an rPFS event in the talazoparib plus enzalutamide group, compared to 53% in the placebo plus enzalutamide group. The median rPFS was not reached in the talazoparib plus enzalutamide arm versus 19.3 months in the placebo plus enzalutamide arm (HR=0.51; 95% CI, 0.32–0.80; P=0.0034). rPFS in the all-comers population who received prior docetaxel is outlined in the graphic below.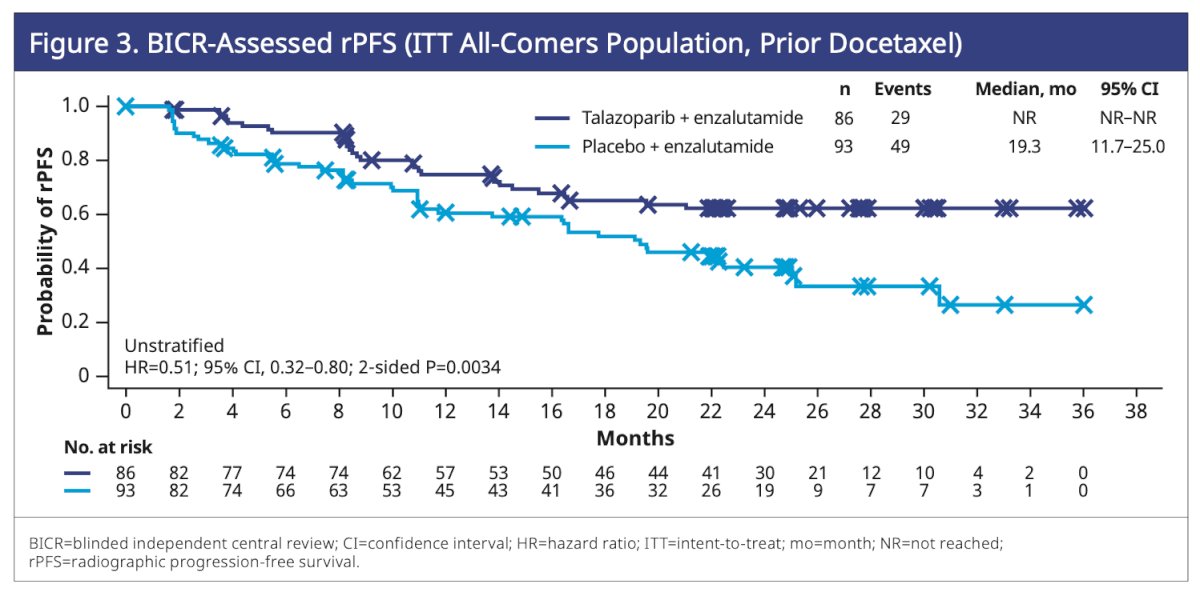
Among HRR-deficient patients who received an ARPI in the hormone-sensitive setting, the median rPFS was more than double in the talazoparib plus enzalutamide arm (13.7 months) compared to the placebo plus enzalutamide arm (5.6 months) (HR=0.53; 95% CI, 0.20–1.42; P=0.20).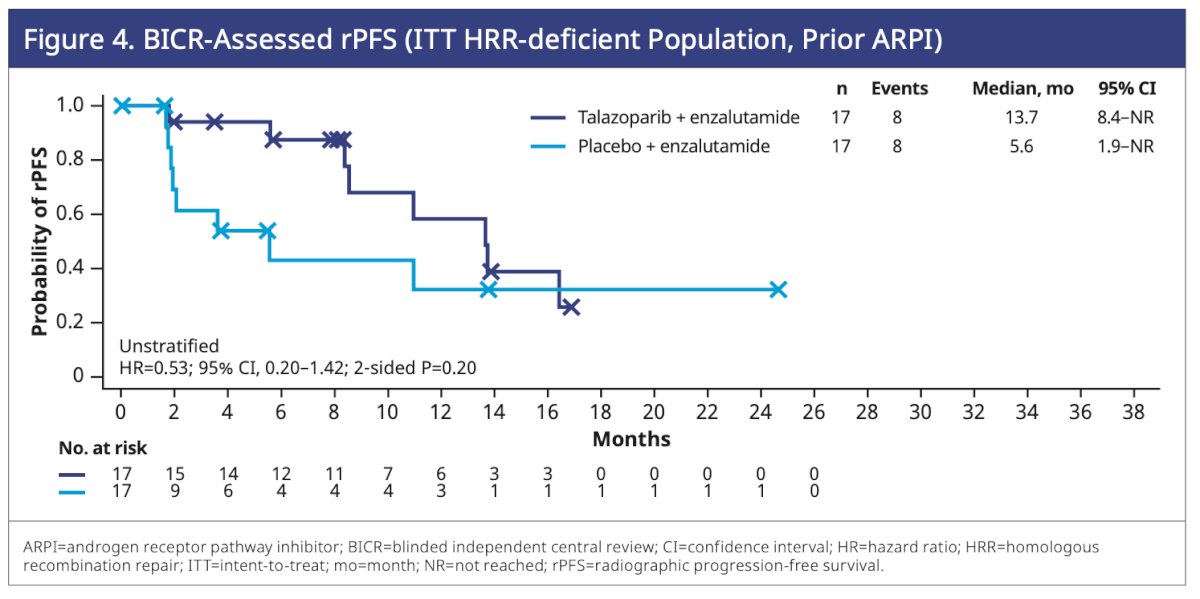
In patients with HRR mutations who previously received docetaxel, the hazard ratio for rPFS was 0.39 (95% CI, 0.22–0.69; P=0.0008), favoring those treated with talazoparib plus enzalutamide.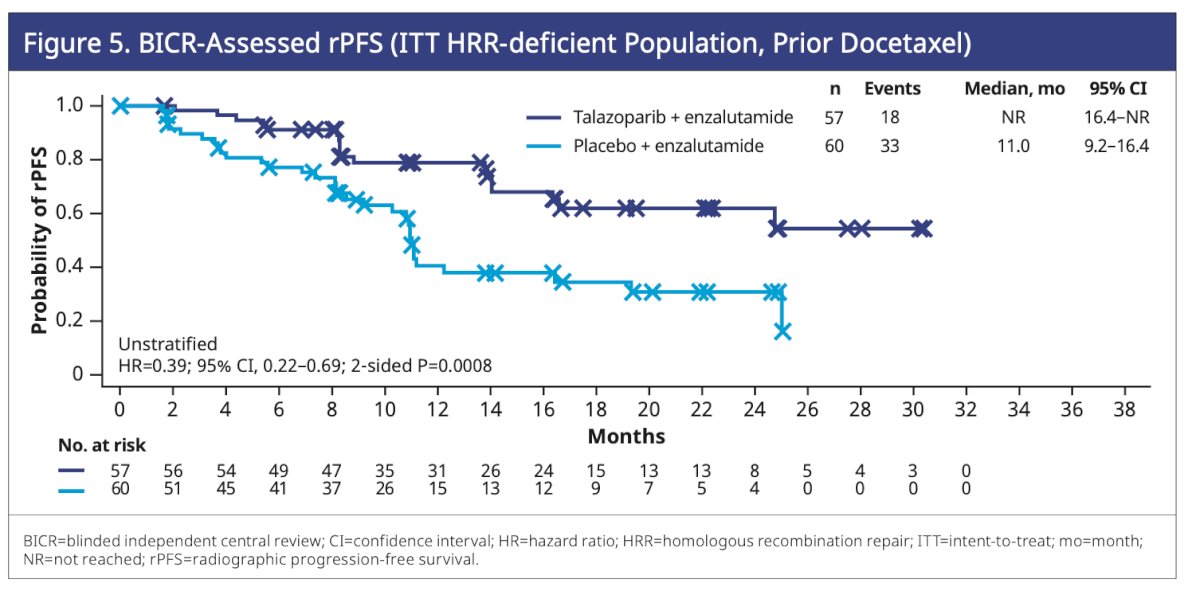
Dr Agarwal wrapped up his poster presentation with the following considerations:
- This post hoc subgroup analysis of TALAPRO-2 confirmed an improved trend in rPFS with talazoparib plus enzalutamide compared to placebo plus enzalutamide, both in patients pretreated with an ARPI or docetaxel in the castration-sensitive setting.
- This benefit is consistent with the advantages observed in both the all-comers population and the HRR-deficient populations of the TALAPRO-2 trial.
- However, it is important to note that the limited number of patients previously treated with an ARPI or docetaxel may have influenced these results.
Presented by: Neeraj Agarwal, MD, FASCO, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
Related coverage: TALAPRO-2 Analysis: Talazoparib Enzalutamide Combo in Pretreated mCRPC - Neeraj Agarwal


