(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the session Poster 1599 presentation. Dr. Karim Fizazi presented the third interim analysis of PSMAfore exploring symptomatic skeletal events, health-related quality of life, and pain in taxane-naive patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC).
177Lu-PSMA-617 is a prostate-specific membrane antigen (PSMA)-targeted radioligand therapy that delivers β-particle radiation to PSMA-expressing cancer cells and the surrounding microenvironment. The VISION study demonstrated the efficacy of 177Lu-PSMA-617 in patients with mCRPC who had previously been treated with at least one androgen receptor pathway inhibitor (ARPI) and one or two taxanes.1
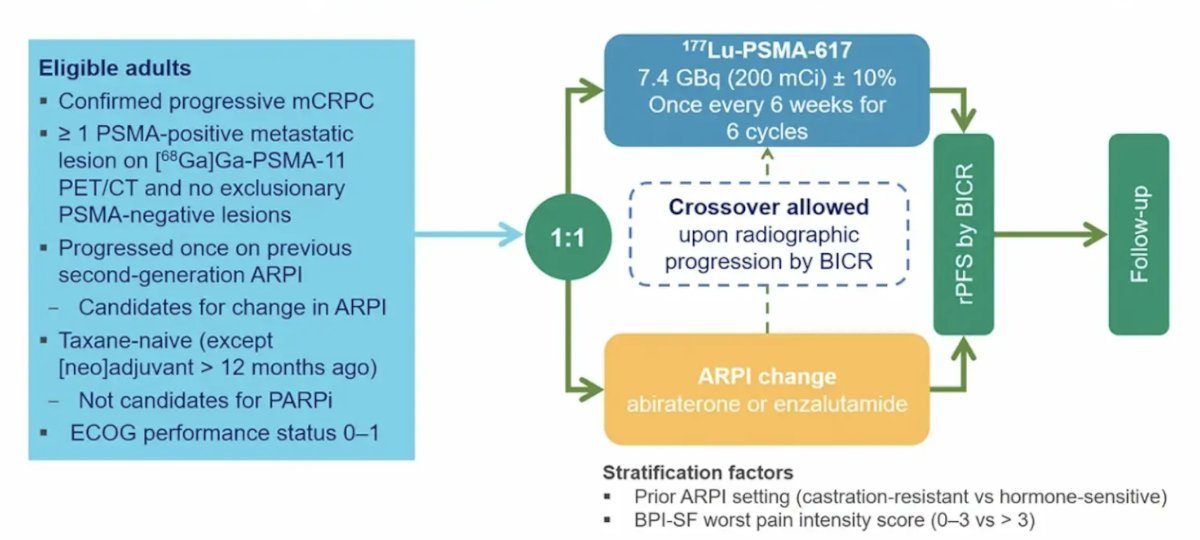
PSMAfore (NCT04689828) a phase 3 trial of 177Lu-PSMA-617 in taxane-naive patients with mCRPC versus androgen receptor pathway inhibitor (ARPI) change in taxane-naive patients with PSMA-positive mCRPC, included patients with mCRPC, candidates for ARPI change after one progression on prior ARPI, and those with ≥1 PSMA-positive lesion and no exclusionary PSMA-negative lesions as determined by 68Ga-PSMA-11 PET/CT. Candidates for PARP inhibition and patients with prior systemic radiotherapy (within the last 6 months), immunotherapy (except sipuleucel-T), or chemotherapy (except [neo]adjuvant therapy administered more than 12 months ago) were ineligible.
Patients were randomized 1:1 to receive either open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or an ARPI change (abiraterone or enzalutamide). Importantly, patients randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression. The trial design for PSMAfore is illustrated in the figure below.
177Lu-PSMA-617 prolonged radiologic progression-free survival (rPFS) compared to ARPI change in taxane-naive patients with PSMA-positive mCRPC, as reported by Dr. Oliver Sartor at the ESMO 2023 annual meeting. Furthermore, secondary and exploratory endpoints, including PSA response, objective response rate (ORR), time to symptomatic skeletal events (SSE), and time to worsening in health-related quality of life (HRQoL) and pain, also favored 177Lu-PSMA-617 at the second interim analysis.
Dr. Fizazi presented the time to symptomatic skeletal events (SSE) and time to worsening (TTW) HRQoL and pain at the third interim analysis (data cutoff, 27 Feb 2024). Data came from self-reported HRQoL (Functional Assessment of Cancer Therapy-Prostate [FACT-P], EQ-5D-5L) and pain (Brief Pain Inventory-Short Form [BPI-SF]). The patient-reported outcome (PRO) questionnaires were completed, and SSEs were monitored throughout treatment and at the end of treatment. The table below illustrates the time-to-event endpoint definitions pro PROs and SSEs.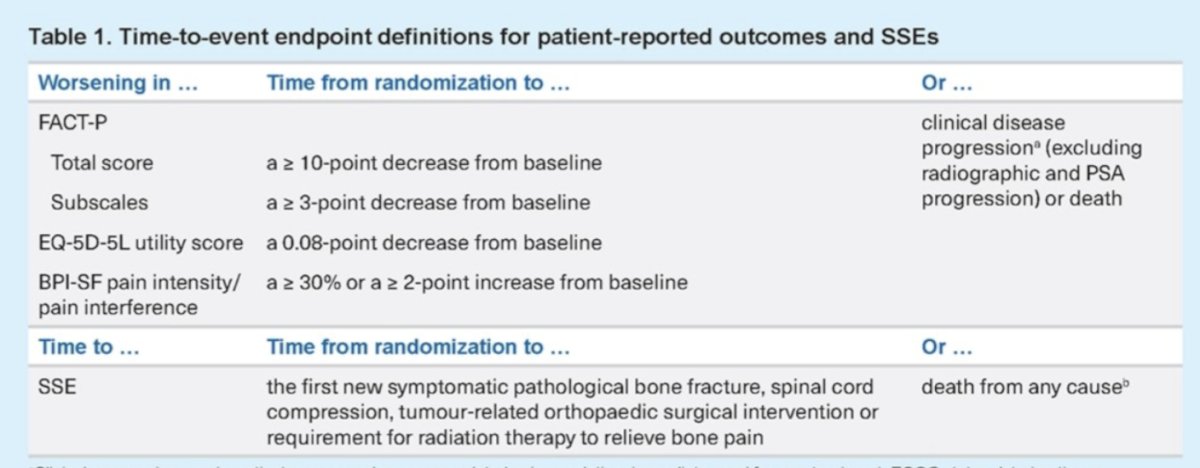
In total, 468 patients were randomized, with 234 in the 177Lu-PSMA-617 arm and 234 in the ARPI change arm. Of these, 97% were treated in the 177Lu-PSMA-617 arm and 99.1% in the ARPI change arm. Baseline characteristics were well balanced between each arm of the trial, with a median age of 72 years (range: 43–94):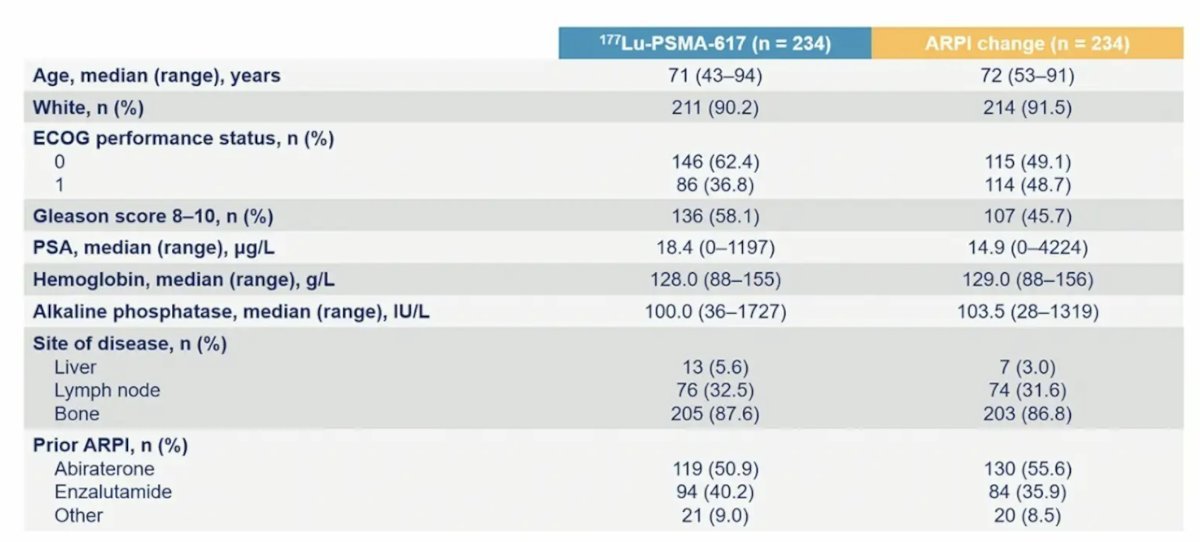
The Median duration of exposure was 8.4 months for 177Lu-PSMA-617 and 6.5 months for ARPI change. Dr. Fizazi reported that 177Lu-PSMA-617 prolonged time to SSE and fewer bone fractures were reported (4 [1.7%]) versus ARPI change (13 [5.6%]). Details of all SSE are shown in the table below:![77Lu-PSMA-617 prolonged time to SSE and fewer bone fractures were reported (4 [1.7%]) versus ARPI change (13 [5.6%])](/images/com-doc-importer/173-esmo-2024/esmo-2024-symptomatic-skeletal-events-health-related-quality-of-life-and-pain-in-a-phase-3-study-of-177lu-lu-psma-617-in-taxane-naive-patients-with-psma-positive-metastatic-castration-resistant-prostate-cancer-third-interim-analysis-of-psmafore/image-3.jpg)
Treatment with 177Lu-PSMA-617 was associated with 62% lower risk of SSE compared to ARPI change (HR 0.38, 95% CI 0.24, 0.61).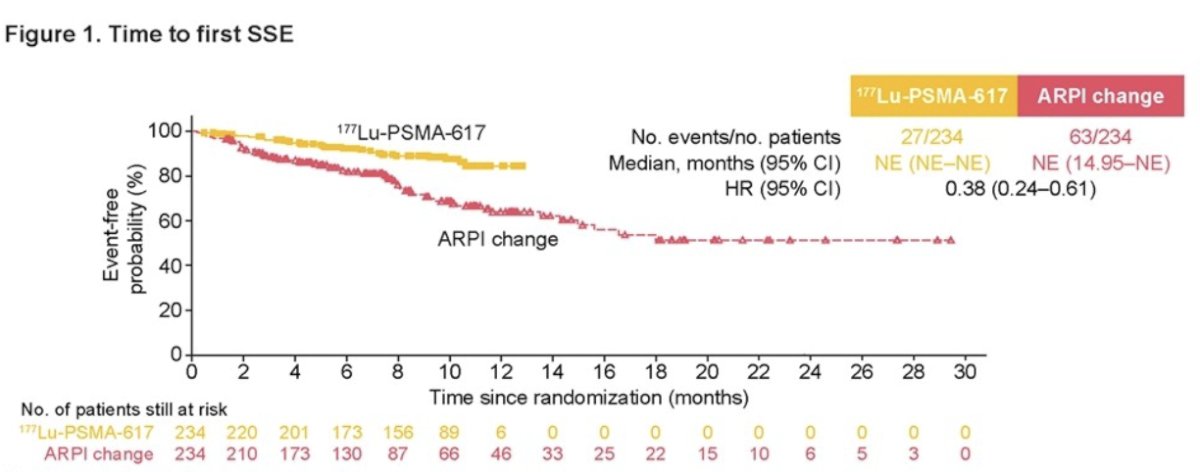
177Lu-PSMA-617 prolonged time to worsening (TTW) in health-related quality of life (HRQoL) compared to ARPI change. This was summarized by the FACT-P total score (HR 0.61, 95% CI 0.50-0.75), and the EQ-5D-5L utility score (HR 0.67, 95% CI 0.54-0.82).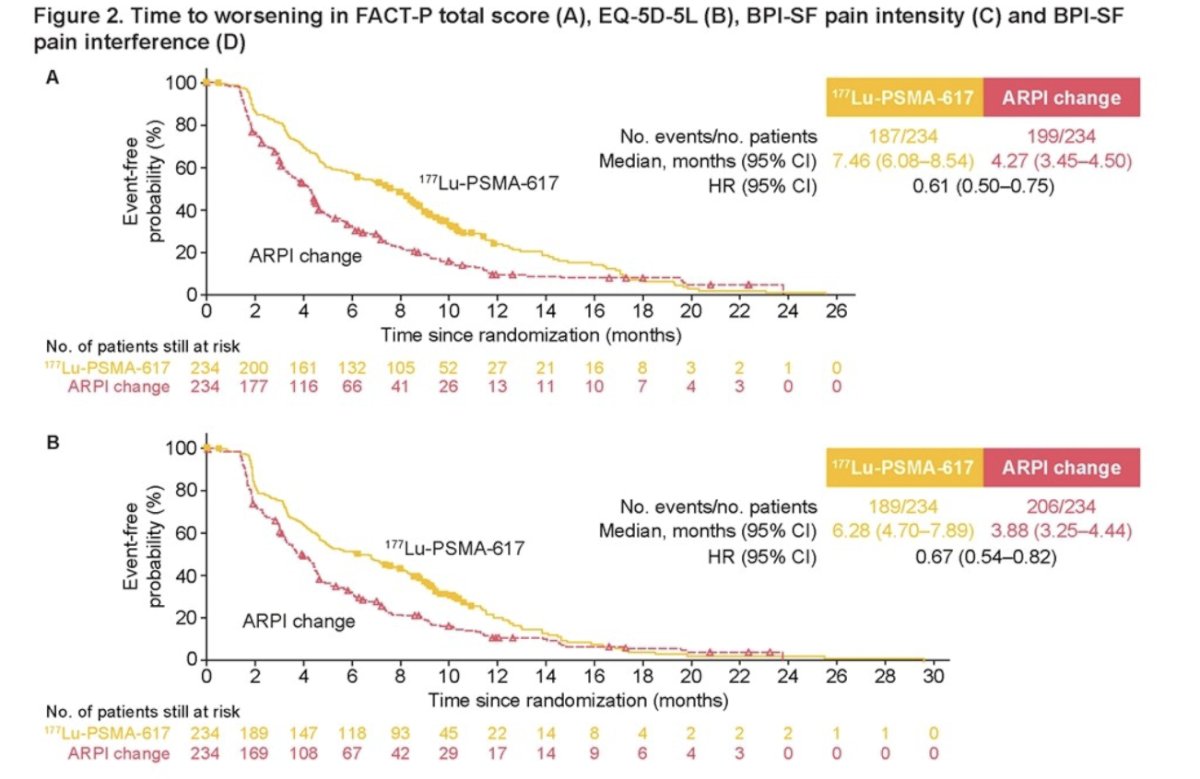
Similarly, 177Lu-PSMA-617 had a lower risk of pain worsening on the BPI-SF pain intensity scale (HR 0.72, 95% CI 0.59-0.88) or pain interference with (HR 0.74, 95% CI 0.61-0.91).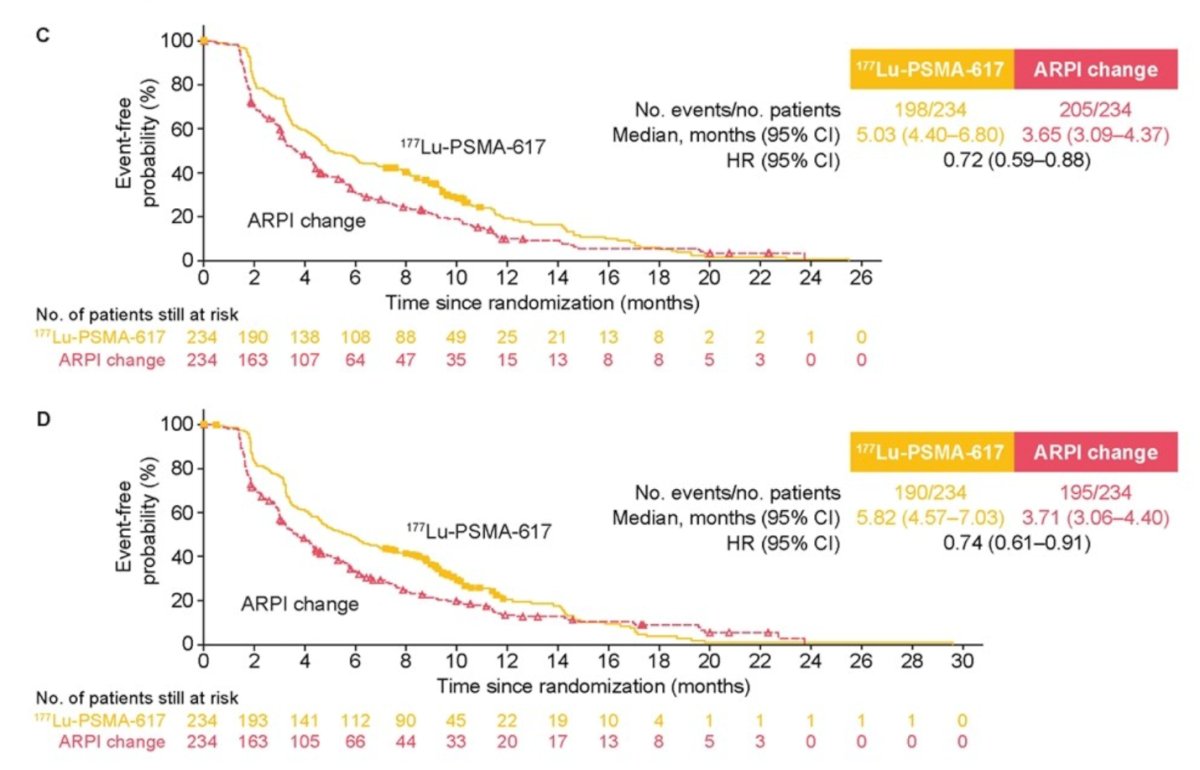
Moreover, when exploring different pain indices, domains, and subdomains of the FACT-P, EQ-5D-5L, and BPI-SF. 177Lu-PSMA-617 was associated with a prolonged TTW in HRQoL in all domains or pain progression as outlined in the forest plot below: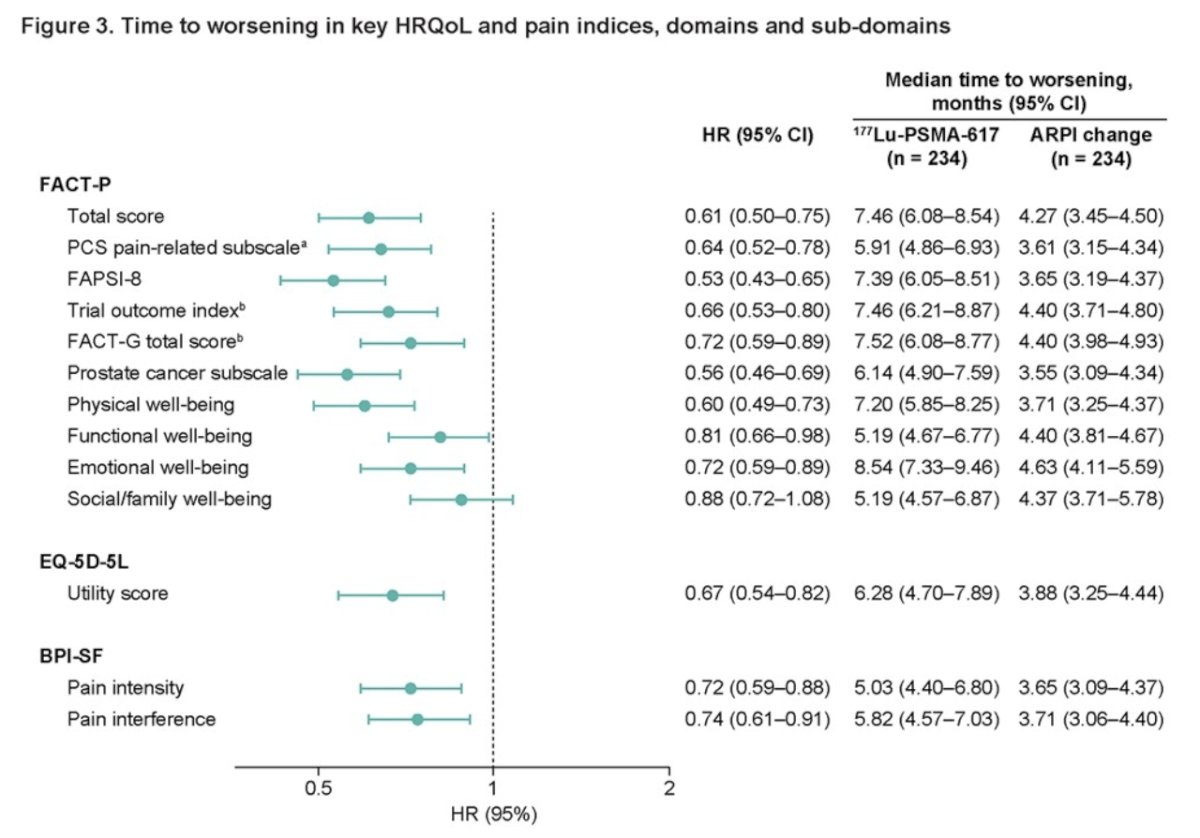
Treatment-related adverse events (TRAEs) grade ≥3 were recorded in 36% in the 177Lu-PSMA-617 and 48% in the ARPI change arm. Serious TRAEs and TRAEs leading to discontinuation for 177Lu-PSMA-617 and ARPI change were, 20% and 32%, and 5.7% and 5.2%, respectively.
Dr. Karim Fizazi concluded his presentation with the following take-home points:
- 177Lu-PSMA-617 prolonged rPFS vs ARPI change in taxane-naive patients with PSMA+ mCRPC in the PSMAfore phase III trial
- Time to symptomatic skeletal events, and time to worsening in health-related quality of life and pain, also favored 177Lu-PSMA-617 in self-reported questionnaires.
- 177Lu-PSMA-617 had a manageable safety profile and was well tolerated and overall was similar between the two treatment arms, but likely confounded by high cross-over rate
- These data support 177Lu-PSMA-617 as a treatment option for patients with mCRPC who have undergone androgen receptor pathway inhibitor treatment.
Presented by: Karim Fizazi, MD, PhD, Professor, Department of Medicine, Institut Gustave Roussy, Paris, France
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References


