(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the presentation of Poster 1601. Dr. Joaquin Mateo presented the first results from ZZFIRST. A randomized phase II trial of enzalutamide with or without talazoparib in metastatic hormone-naïve prostate cancer (mHNPC).
Three different trials in the metastatic castration-resistant prostate cancer (mCRPC) space have shown significant improvement in oncological outcomes (rPFS, OS) with the combination of androgen receptor pathway inhibitors (ARPIs) and PARP inhibitors (PARPi) in patients with DNA damage repair (DDR) alterations. There is a close interplay between the androgen receptor (AR) pathway and the DNA damage repair (DDR) machinery, supporting the synergistic effect of this combination. 1-3
ZZ-First is the first multicenter, open-label, randomized, phase II clinical trial in men with metastatic hormone-naïve prostate cancer, comparing enzalutamide (ARPI) with or without talazoparib (PARPi).
The inclusion criteria for this trial were:
- Male ≥18 years.
- Histologically confirmed adenocarcinoma of the prostate without predominance of small-cell or neuroendocrine features
- High-volume metastatic disease documented on bone scan or CT/MRI.
- Life expectancy ≥ 12 months.
- Prior androgen deprivation therapy (ADT)-based regimen was allowed if progression occurred while on non-castrate testosterone levels > 12 months after discontinuation.
- No prior treatment with enzalutamide, apalutamide, darolutamide or abiraterone acetate.
- PSA ≥ 4 ng/mL at diagnosis or before starting ADT therapy.
- No prior systemic therapy for metastatic prostate cancer.
The primary endpoint was PSA-complete response, defined as PSA <0.2 ng/mL after 12 months of therapy in patients with mHNPC in the ADT + enzalutamide + talazoparib arm. Secondary endpoints included PSA-complete response at any time point, at month 7 and month 12, PSA-progression free survival (PFS), radiologic PFS, overall survival, and safety and tolerability.
Briefly, this trial identified 54 patients with mHNPC who received ADT + enzalutamide. After 8 weeks, patients were randomized 1:2 into either the control arm (ADT + enzalutamide) or the interventional arm (talazoparib + enzalutamide + ADT). Patients were followed until progression, unacceptable toxicity, death, or discontinuation of study drugs. The study diagram is shown below.
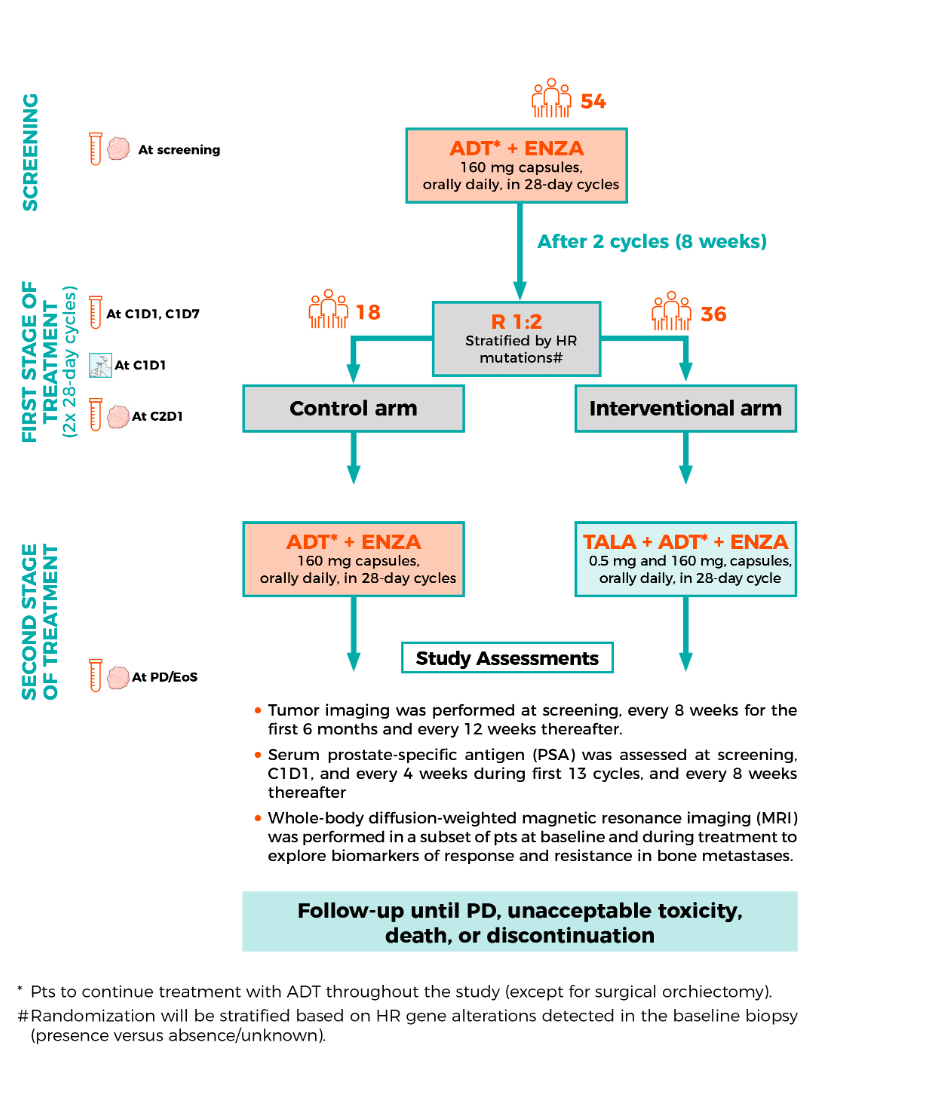
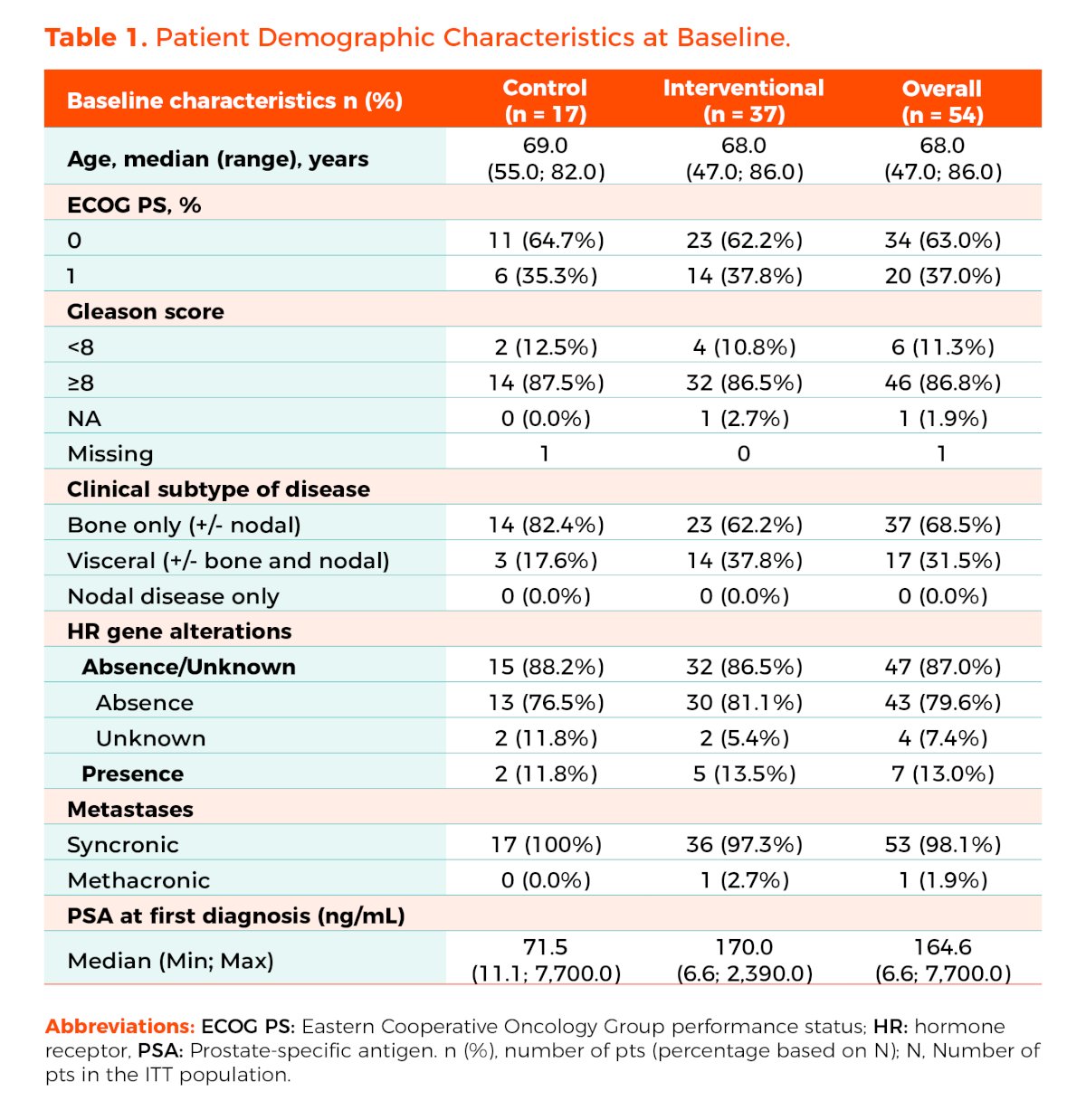
Dr. Mateo presented that baseline patient characteristics were well balanced between both groups. In the overall population, the median age was 68 years. Most patients (63%) had an ECOG-PS of 0, 87% had a Gleason score of ≥8, and 98% had synchronous metastases or de novo disease. Additionally, 13% had homologous recombination repair (HRR) gene alterations.
With a cutoff date of February 2024, 19 (51.4%) of patients in the intervention arm vs. 9 (52.9%) in the control arm were still receiving the treatment they had been allocated to. The median follow-up for this presentation was 30.6 months.
The primary endpoint, PSA-complete response, in the interventional arm was achieved in 73% (95% CI, 55.9%-86.2%, p<0.001), compared to 64.7% in the control arm. The radiologic PFS was not significantly different between the treatment groups. 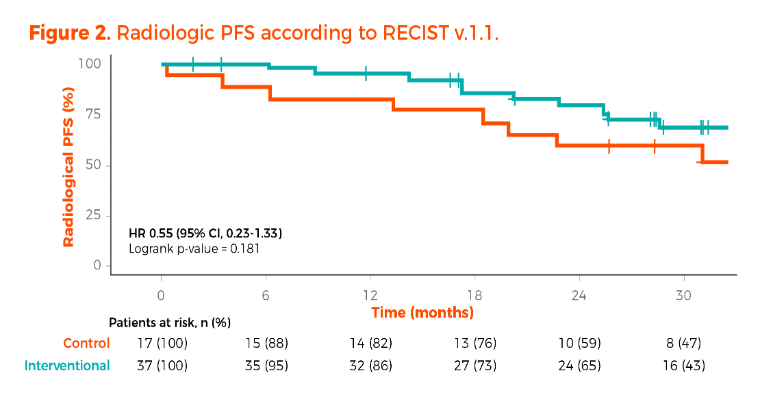
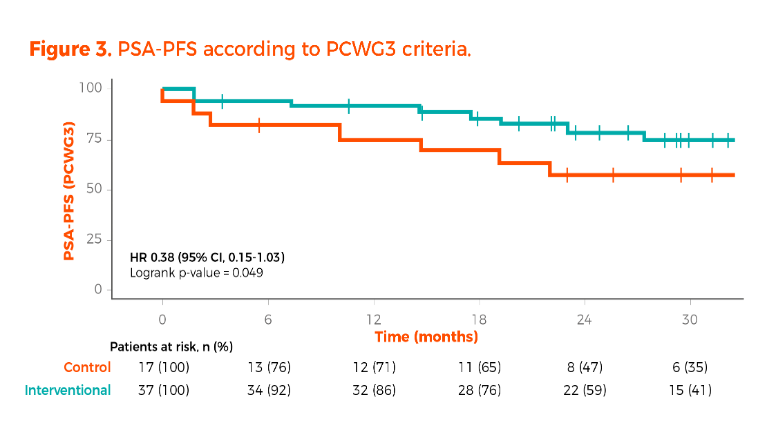
The PSA-PFS according to the Prostate Cancer Working Group 3 (PCWG3) criteria significantly favored the interventional arm (log-rank p value 0.049). However, the 95% confidence interval crossed the line of 1 (HR 0.38, 95% CI, 0.15-1.03).
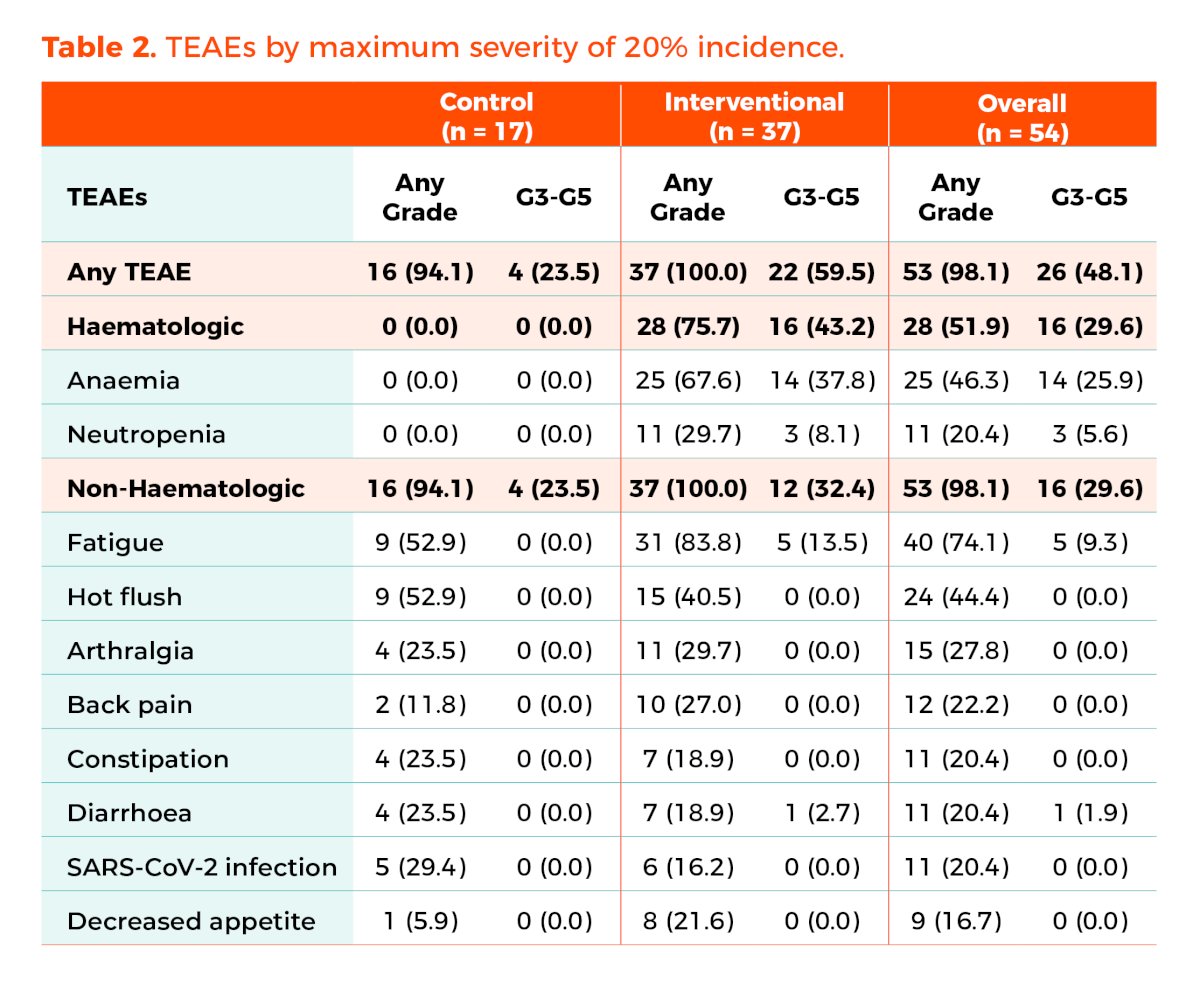
There were no major safety concerns. Treatment-related deaths occurred in two patients in the interventional arm who developed acute leukemia after 26 and 32.8 months on treatment. Tolerability was not an issue; however, the dose of talazoparib was reduced in 14 of the 37 (37.8%) patients in the interventional arm. The dose of enzalutamide was reduced in 1 patient (5.9%) in the control arm and in 3 patients (8.1%) in the investigational arm. Anemia of any grade was observed in 67.6% of patients in the interventional arm, with ≥ Grade 3 anemia documented in 38% of patients.
Dr. Mateo mentioned that the next steps for this trial are to continue follow-up until mature rPFS, PSA-PFS, and OS data can be analyzed. They plan to present the study's baseline and +2 months biopsies (transcriptomics) to analyze changes in the DDR pathway upon enzalutamide treatment. Additionally, they will monitor ctDNA kinetics and subclonal dynamics at response and progression and delve into the molecular landscape upon progression to CRPC post-intensified therapy in mHNPC.
Dr Mateo concluded his poster presentation with the following take home messages:
- In this initial report from ZZ-First, the PSA-complete response in the interventional arm and the control arm (73% vs. 65%) was similar
- Patients will need to be followed for longer to obtain more mature rPFS, PSA-PFS, and OS data.
- There are no major safety signals or concerns in the interventional arm, though anemia remains associated with the use of PARPi.
- The dose of talazoparib needed to be reduced in 38% of patients, but overall tolerability appears to be acceptable.
Presented by: Joaquin Mateo, MD, PhD, Physician-Scientist and Medical Oncologist, Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
Related content: ZZ-FIRST Trial Demonstrates High PSA Response with Combination Therapy - Joaquin Mateo
2. Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Final overall survival (OS) in PROpel: Abiraterone (abi) and olaparib (ola) versus abiraterone and placebo (pbo) as first-line (1L) therapy for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2023; 41: LBA16-LBA16.
Related Content:
ZZ-FIRST Trial Demonstrates High PSA Response with Combination Therapy - Joaquin Mateo


