(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the presentation of poster 1641. Dr. Christos Kyriakopoulos presented the results of a phase 1/2 trial of oral Masofaniten (EPI-7386) in combination with Enzalutamide compared to Enzalutamide alone in metastatic castration-resistant prostate cancer (mCRPC) patients.
Oral Masofaniten (EPI-7386) is a next-generation aniten designed to inhibit androgen receptor (AR) activity by binding to the N-terminal domain. Unlike other androgen receptor pathway inhibitors (ARPIs) such as enzalutamide, apalutamide, or darolutamide, which target the ligand-binding domain of the AR, Masofaniten binds to a different area. This binding mechanism means it is not affected by resistance mechanisms associated with the ligand-binding domain, including point mutations or truncated splice variants of the AR. Preclinical data supports its efficacy in disrupting AR-regulated gene transcription, even in the presence of these resistance mechanisms. The action mechanism of the Aniten is illustrated in the graphic below:
_0)
Preclinical RNAseq and CHIPseq data suggest that the combination of EPI-7386 plus Enzalutamide results in a deep blockade of the androgen receptor pathway with greater antitumor activity compared to Enzalutamide alone. Dr. Kyriakopoulos presented the results of the phase 1 trial, while the phase 2 is currently enrolling in the USA, Canada, Australia, France, Belgium and Spain. This Phase 1/2 multicenter, open-label clinical trial (NCT05075577) examines the combination of EPI-7386 plus enzalutamide in mCRPC patients on ADT, but naïve to prior ARPIs, however, one line of prior chemotherapy in the metastatic hormone-sensitive setting was allowed for inclusion.
The phase 1 part of this trial examined escalating doses of masofaniten + enzalutamide at 120 or 160 mg once daily doses. The primary and secondary endpoints of the phase 1 portion were to evaluate the safety and pharmacokinetics of masofaniten + enzalutamide when co-administered together, and to establish the recommended phase 2 combination dose (RP2D). The phase 2 portion of this trial was designed as a two arm, 2:1 randomized trial evaluating the anti-tumor activity of masofaniten with enzalutamide versus enzalutamide alone.
The phase 1 of the trial completed enrollment with 18 patients in four cohorts. A total of 16 patients were evaluable for efficacy, as per protocol and 12 are still ongoing. The RP2CD was established at masofaniten 600 mg BID + Enzalutamide 160 mg QD. For the phase 2, approximately 120 patients were planned for randomization to masofaniten 600 mg twice daily + enzalutamide 160 mg once daily (n=80) versus single agent enzalutamide at 160 mg once daily (n = 40) as illustrated below:
_1)
A Total of 14/18 patients enrolled in Phase 1 presented ≥ 2 risk factors of early treatment failure on enzalutamide single agent. Risk factors were: LDH ≥ULN, ALP ≥ULN, Albumin <35g/L, de novo metastatic disease at diagnosis, <3 years since initial Dx, >5 bone metastases, presence of visceral metastasis, PSA doubling time <2.8 months, opioid use >14 days. Baseline characteristics of the Phase 1 trial are shown below:
_2)
The combination regimen (EPI-7386+Enzalutamide) was well-tolerated with a safety profile consistent with enzalutamide monotherapy. There was one grade 3 rash observed in cohort 4 (masofaniten 600 mg twice daily + enzalutamide 160 mg once daily). The most common treatment related adverse events (TRAEs) were fatigue, hypertriglyceridemia, hypercholesterolemia and diarrhea. In Cohort 4, one Grade 3 rash, maculo-papular event was reported, this was deemed as probably related to the combination treatment and was managed with a dose modification.
_3)
Pharmacokinetics results showed that enzalutamide exposure was not affected by the concomitant administration of Masofaniten, permitting the use of the full dose of enzalutamide (160 mg). Conversely, Masofaniten exposure needed to be consistently reduced when administered with enzalutamide, due to enzalutamide's known role as a CYP3A4 inducer, which metabolizes Masofaniten. Despite this, Masofaniten levels remained within the active range observed in preclinical studies, with the highest exposures and Cmin achieved with twice-daily dosing.
_4)
_5)
To date, with a median follow up of 15.2 months, >60% of the patients are still on study. 18 patients were enrolled and 11 are ongoing treatment. Patient disposition in the study is illustrated below:
_6)
A total of 14 out of 16 patients (88%) achieved a PSA90 response, while 14 out of 16 (88%) achieved a PSA50 response. Additionally, 10 out of 16 patients (63%) reached a PSA level of <0.2. Rapid, deep, and durable PSA responses were observed regardless of initial PSA levels, combination dose/regimen, or prior chemotherapy status (6/16 evaluable patients received prior docetaxel in the mHSPC setting).
_7)
Of the patients who had measurable disease 3/5 had an overall response to treatment, 60%, achieved a partial response (PR) and 2/5 (40%) had stable disease as illustrated below.
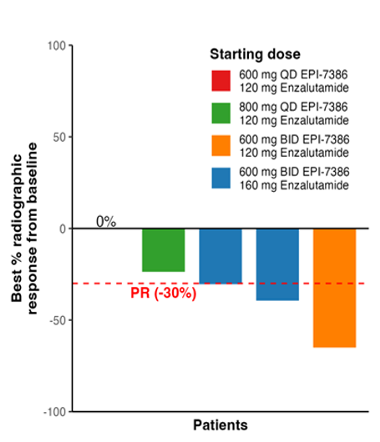
Dr. Kyriakopoulos reported that the Phase 1 data on masofaniten plus enzalutamide is still immature for time-to-event analysis. However, the data compares favorably with other first-line mCRPC trials, such as the AFFIRM and PREVAIL trials, regarding time to pain progression, radiologic progression-free survival, and progression-free survival.
_9)
Dr. Kyriakopoulos concluded his poster presentation with the following take home messages:
- The combination of masofaniten and enzalutamide at all dose/schedule regimens tested in the Phase 1 component of the study continues to be well tolerated and efficacy parameters are proving to be durable in this patient population
- Of note, 78% of mCRPC patients enrolled in the Phase 1 part presented 2 or more risks of early failure to enzalutamide single agent treatment.
- A total of 88% of the patients dosed with masofaniten + enzalutamide achieved a PSA decline > 90%
- With a current median follow up of 15.2 months, time to event parameters continue to compare favorably with historical data (AFFIRM, PREVAIL) of single agent enzalutamide
- The Phase 2 component of the study is currently open to enrollment at approximately 33 sites in the US, Canada and Australia.
Presented by: Christos Kyriakopoulos, MD Medical Oncologist, Associate Professor University of Wisconsin, United States of America.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
Related content: Masofaniten in Prostate Cancer Phase 1/2 Trial Data and Future Directions - Christos Kyriakopoulos


