(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a presidential symposium of practice-changing trials. Professor Karim Fizazi discussed the results of EORTC-GUCG 1333/PEACE-3, a phase III trial of radium-223 plus enzalutamide in asymptomatic or mildly symptomatic patients with bone metastatic castrate-resistant prostate cancer (mCRPC)
Dr. Fizazi began by highlighting the efforts of the PEACE consortium (The ProstatE cAncer Consortium in Europe) which aims to conduct collaborative phase III trials for prostate cancer in Europe. The primary sponsor can be any academic entity (group, hospital, university). Study budgets are assigned on a per trial basis with funds supported by public grants, pharmaceutical industry support, and/or charities. Between 2015 and 2024, 8 phase III trials have been activated, and 3 have completed accrual, with >3,000 patients randomized.
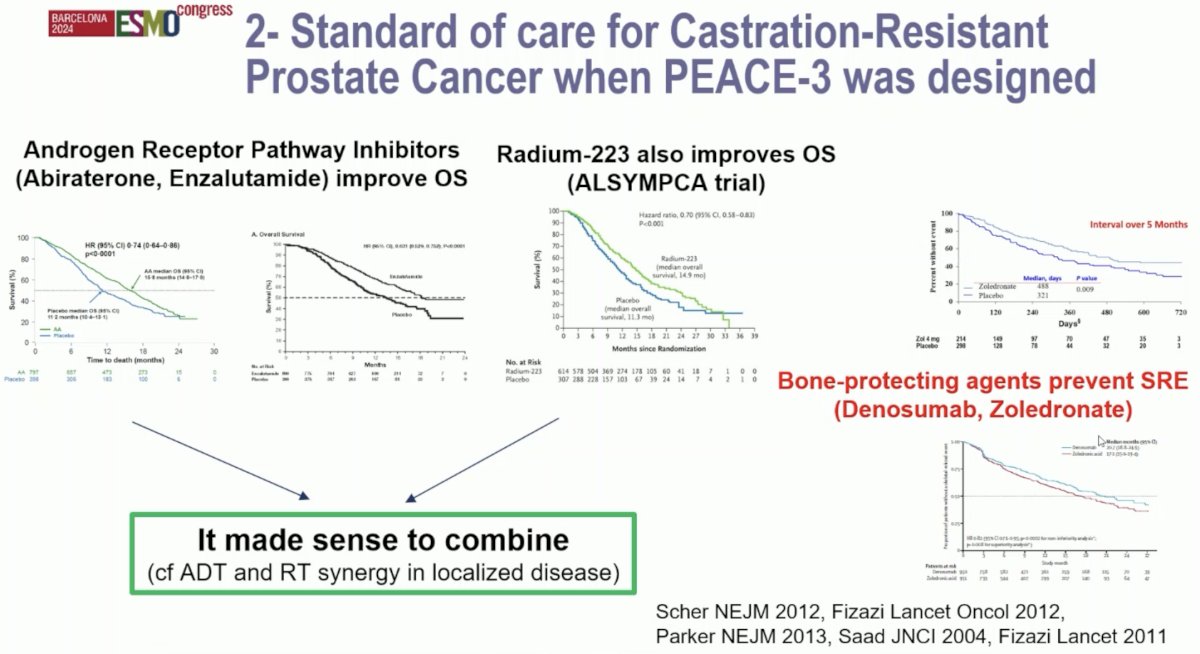
What was the study rationale for PEACE-3? Both androgen receptor pathway inhibitors (i.e., enzalutamide, abiraterone) have been shown to improve overall survival.1,2 Similarly, 223Ra was shown to improve overall survival in minimally or asymptomatic mCRPC patients with bone metastases.3 Furthermore, the combination of radiotherapy and ADT has been shown to have synergistic effects for the primary treatment of patients with clinically localized disease. An often-forgotten component of the treatment paradigm for these patients is the use of bone-protecting agents to prevent symptomatic skeletal-related events, with both denosumab and zoledronic acid having shown benefits in preventing such events.4,5
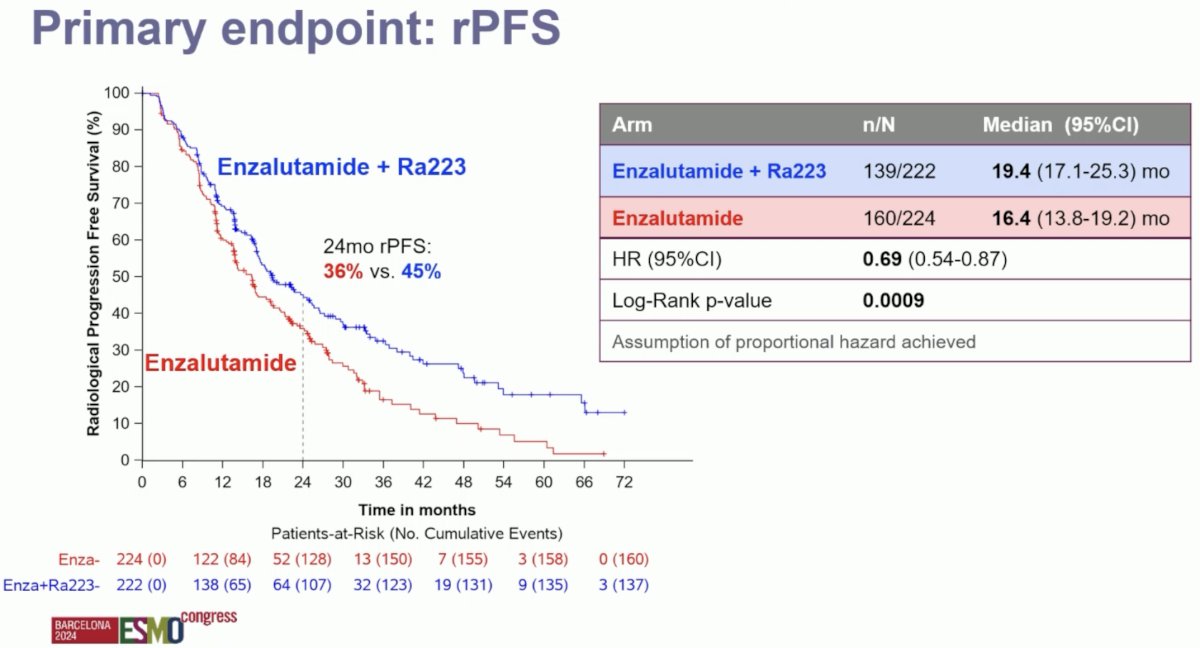
The PEACE-3 trial met its primary endpoint. The addition of 223Ra to enzalutamide was associated with significant improvements in radiographic progression-free survival (median: 19.4 versus 16.4 months; HR: 0.69, 95% CI: 0.54–0.87, p=0.0009). At 24 months, 45% of patients in the 223Ra combination arm were free of radiographic progression, compared to 36% of patients in the enzalutamide monotherapy arm.
However, Dr. Fizazi noted that there are important limitations to the use of radiographic progression-free survival as an endpoint:
- It does not directly measure clinical benefit
- It is rarely routinely measured in practice
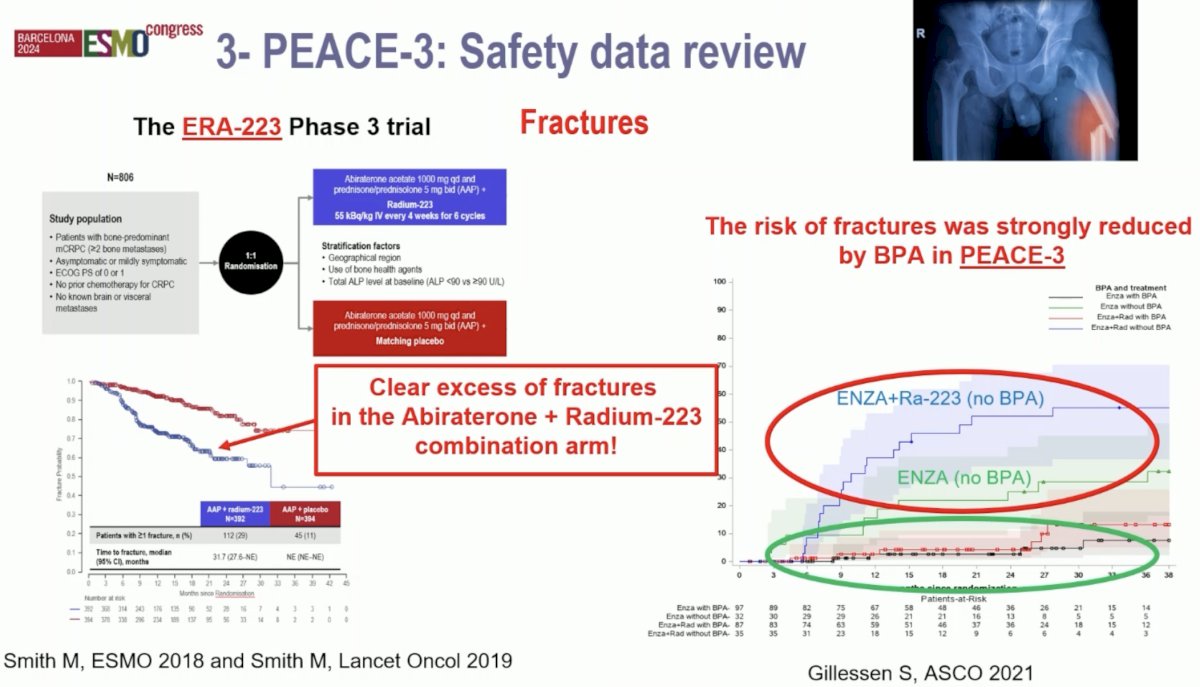
Given the increased rate of skeletal fractures reported in the ERA-223 trial with combination abiraterone + 223Ra, a study amendment was introduced following the enrollment of the initial 119 patients to mandate the use of bone protecting agents in the PEACE-3 trial. The impact of this amendment is illustrated in the figure below. Encircled in red below are the rates of fractures in each treatment arm prior to implementing the requirement for bone protecting agents. Following this amendment, we see that the fracture rates significantly decreased in both arms.
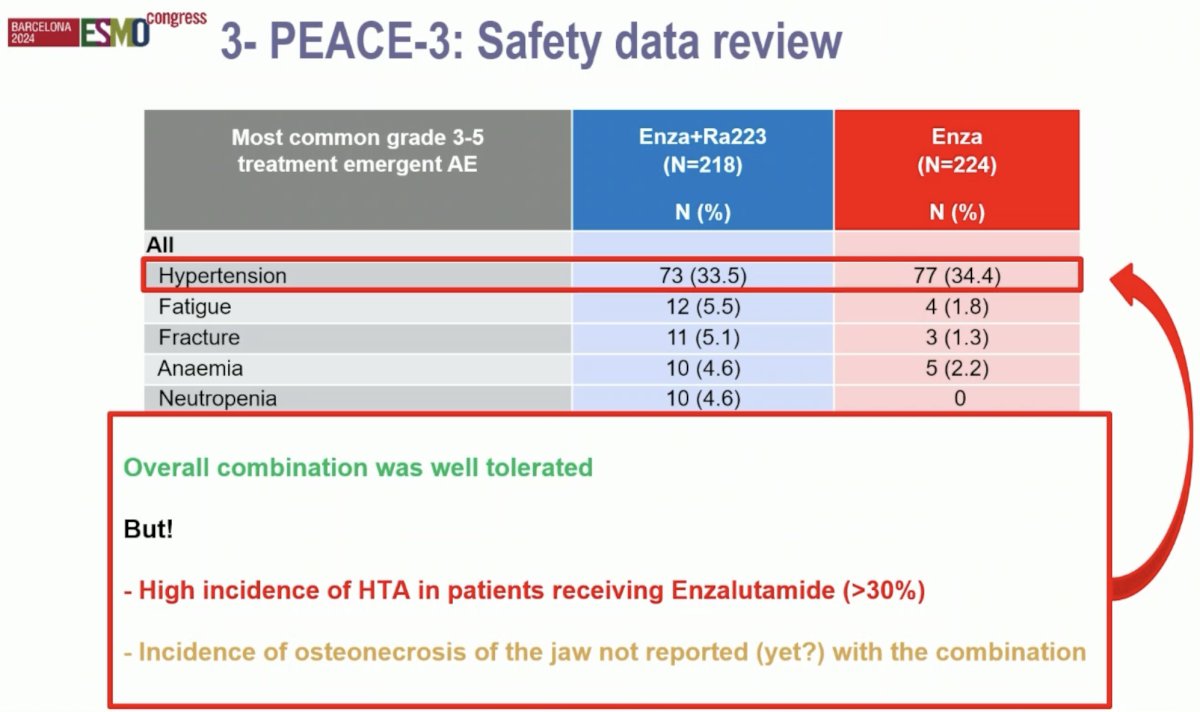
From a safety standpoint, Dr. Fizazi noted that there were no new or worrisome safety signals. He highlighted the high incidence of grade ≥3 hypertension (~34%) in both treatment arms, which treating physicians must be aware of and address. To date, the incidence of osteonecrosis of the jaw has not yet been reported for the combination of 223Ra + enzalutamide.
Are the results of PEACE-3 practice changing? He argued that a radiographic progression-free survival benefit alone is not sufficient. He iterated his belief in the concept of ‘rPFS’ + another meaningful endpoint to justify the ‘practice changing’ moniker. In the PEACE-3 trial, time to pain progression and symptomatic skeletal events were similar for both arms. However, an overall survival benefit was observed, which meets the ‘another’ criterion. The combination of 223Ra + enzalutamide was associated with overall survival improvements from 35 to 42.3 months (HR: 0.69, 95% CI: 0.52–0.90). However, there was evidence of non-proportional hazards, and, as such, the study statisticians recommended that the investigators wait for the final overall survival analysis to determine whether there is a significant overall survival benefit. Dr. Fizazi argued however that the observed overall survival difference is already clinically meaningful, and we do not necessarily need to wait for a significant statistical difference.
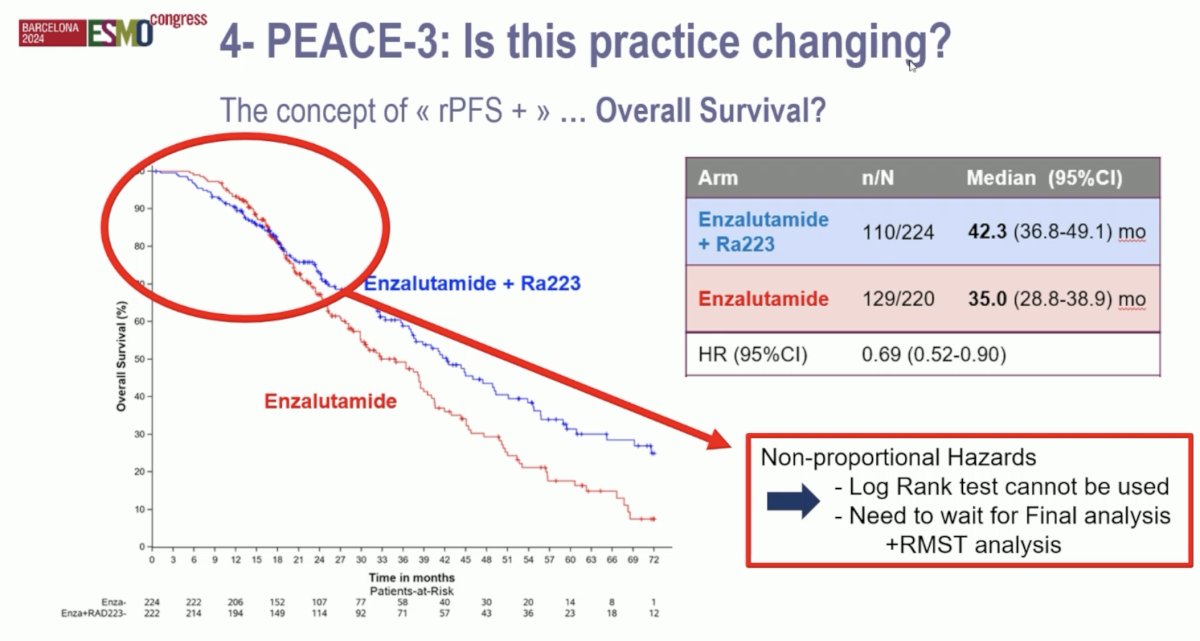
Thus, Dr. Fizazi argued that the results of PEACE-3 are practice changing, albeit with the caveats of pending long-term overall survival analysis and data on osteonecrosis of the jaw. The ESMO guidelines will likely be amended soon to reflect this combination as a potential new standard of care. It is important to note that this study combination is not a double combination, but practically speaking, is at least a quadruplet combination of:
- Enzalutamide
- Radium-223
- Denosumab (or zoledronic acid)
- Androgen deprivation therapy
- +/- Anti-hypertensive treatment
Are the data useful from a practical standpoint? The current standard of care is using an ARPI earlier (for mCSPC, nmCRPC, etc.) and is no longer reserved for mCRPC patients (like in PEACE-3). However, the reality is that ADT alone remains the treatment of mHSPC patients in many countries, and, thus, the PEACE-3 data are applicable to these situations.
Can this combination be used in the post-abiraterone (i.e., in patients receiving abiraterone in the hormone-sensitive setting)? This was a rare scenario in PEACE-3 (only 2%), and there is thus no evidence yet to support this sequence.
In general, before becoming fully applicable, more data are needed on bone protecting agents used in PEACE-3:
- Was denosumab mostly used in the trial?
- What is the appropriate duration of use? Life-long or only 2-3 years?
- What is the incidence of osteonecrosis of the jaw in PEACE-3? Are there any safety issues when combined with Radium-223?
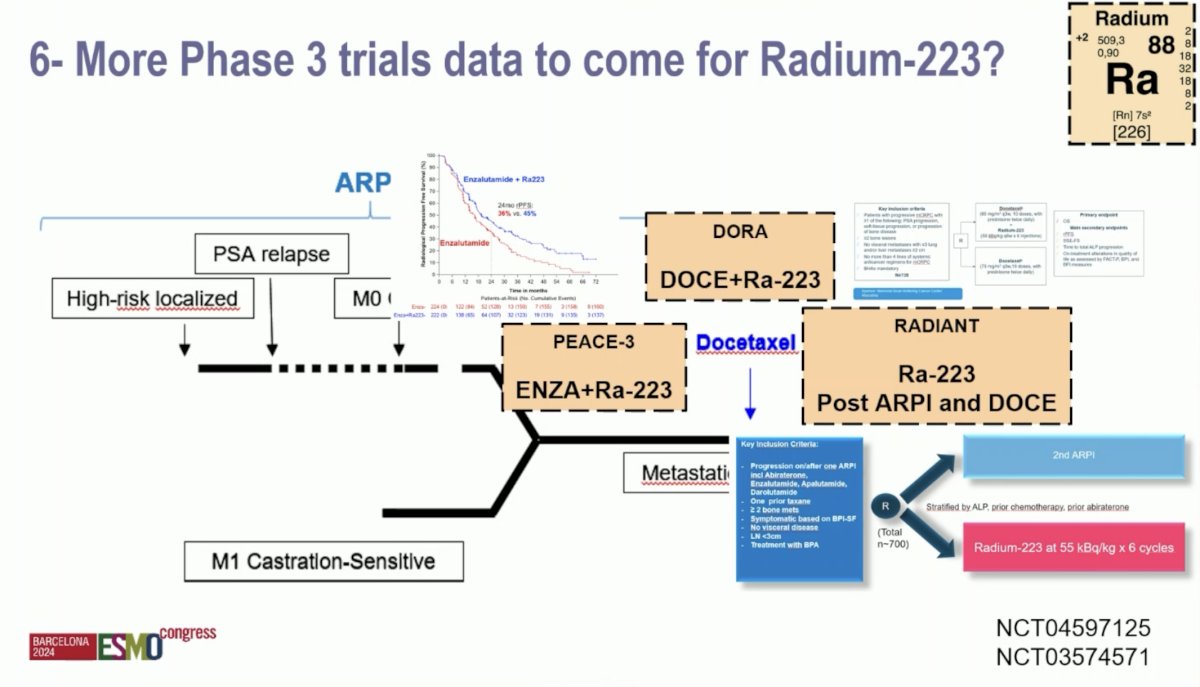
Ongoing phase III trials are evaluating 223Ra in the mCRPC disease space. DORA is evaluating the combination of docetaxel + 223Ra. RADIANT is evaluating 223Ra in the post-ARPI and docetaxel setting.
Dr. Fizazi concluded as follows:
- Combining enzalutamide and Radium-223 improves radiographic progression-free survival (HR: 0.69) and probably also overall survival (HR: 0.69) in (mostly ARPI-naive) mCRPC patients with predominant bone metastases
- If this combination is used, a bone protecting agent is mandatory (mostly denosumab)
- Baseline symptomatic bone disease is not needed to benefit from enzalutamide + 223Ra
- Blood pressure should be monitored when using enzalutamide (+/- Radium-223)
- More data are needed from PEACE-3 (osteonecrosis of the jaw, quality of life, long-term overall survival, etc.)
Presented by: Karim Fizazi, MD, PhD, Professor, Department of Medicine, Institut Gustave Roussy, Paris, France
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368(2):138-48.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-33.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomized, double-blind study. Lancet. 2011;377(9768):813-822.
- Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379(9810):39-46.


