(UroToday.com) The 2024 ESMO annual meeting included a session highlighting prostate cancer trials in progress, with Dr. Sarah Howlett discussing the trial design of STAMPEDE2, a phase 3, randomized, open-label trial of niraparib-abiraterone acetate + prednisolone in patients with metastatic prostate cancer with a deleterious alteration in a HRR gene starting ADT. Treatment intensification at the start of ADT improves overall survival in metastatic prostate cancer.
Further, up to 15% of metastatic prostate cancer harbors deleterious alterations in BRCA2, or related HRR genes that confers sensitivity to PARP inhibitors. Multiple trials have demonstrated the efficacy of PARP inhibitors in metastatic castration-resistant prostate cancer. In addition, there is evidence of a synergistic effect when PARP inhibitors are combined with abiraterone acetate. STAMPEDE2 is designed to reflect real-world treatment practices and will test whether upfront ADT + niraparib + abiraterone acetate + prednisone in HRR-altered hormone-sensitive prostate cancer can improve survival compared to standard-of-care treatment.
STAMPEDE2 is a phase III, randomized, open-label, multi-center platform protocol testing treatments in metastatic hormone-sensitive prostate cancer (NCT06320067, ISRCTN66357938). Metastatic disease should be confirmed on conventional CT and bone imaging. For the niraparib-abiraterone acetate + prednisolone trial, patients identified as biomarker positive (HRR gene alteration) on clinical next-generation tumor sequencing are offered randomization 1:1 to either arm A (standard of care with physician’s choice androgen receptor signaling inhibitor) or arm N (standard of care with niraparib-abiraterone acetate + prednisolone). The trial design is as follows: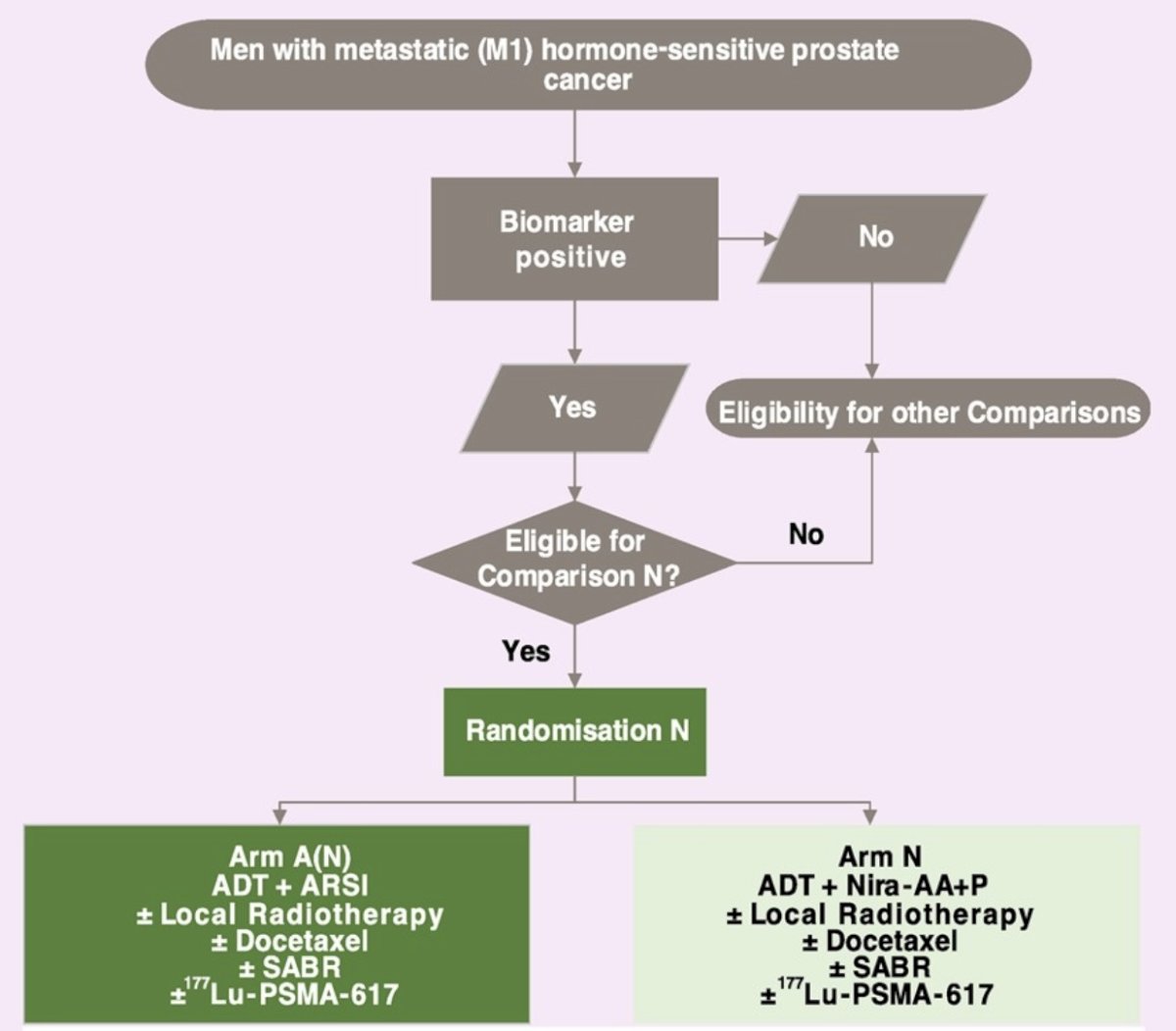
Prior randomization into the STAMPEDE2 SABR or PSMA-Lutetium trials is allowed. The key exclusion criteria, interventions, and the secondary endpoints are as follows: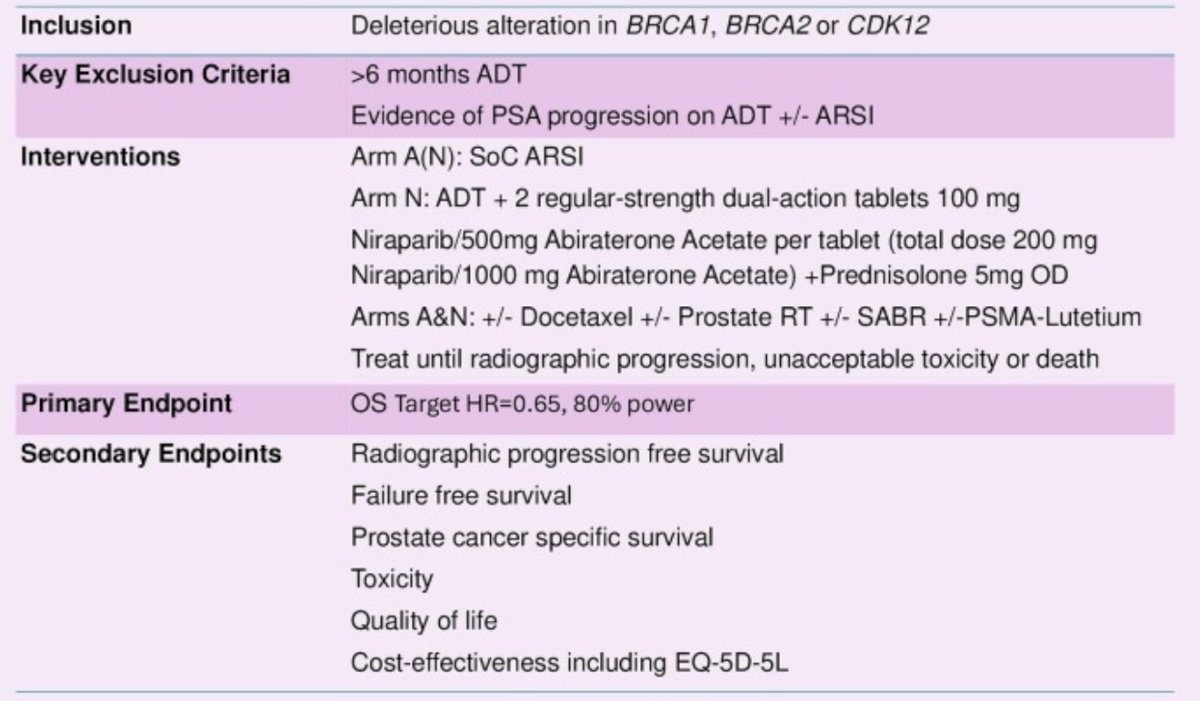
Randomization is stratified by planned use of docetaxel and/or prostate radiotherapy and/or SABR/PSMA-Lutetium. Target recruitment is 680 patients with the primary outcomes overall survival, with a target hazard ratio of 0.65 and 80% power. STAMPEDE2 is open to recruitment in the UK with the potential to open at international sites; the trial is planned to open in ~80 UK sites. Biomarker testing will be carried out locally using pre-existing pathways.
Presented by: Sarah Howlett, Clinical Research Fellow, University College Hospital NHS Foundation Trust, London, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


