(UroToday.com) The 2024 ESMO annual meeting included a session highlighting prostate cancer trials in progress, with Dr. Hoda Abdel-Aty discussing the trial design of STAMPEDE2, a phase 3, randomized, open-label trial of stereotactic ablative body radiotherapy in patients with newly diagnosed oligometastatic prostate cancer starting ADT.
Oligometastatic disease is an intermediary metastatic site, and stereotactic ablative body radiotherapy in metachronous oligometastatic prostate cancer improves progression free survival and defers use of systemic therapy. However, there is limited data that exists regarding the role of stereotactic ablative body radiotherapy in synchronous oligometastatic prostate cancer. The STAMPEDE2 SABR Trial will investigate the addition of stereotactic ablative body radiotherapy to standard of care in newly diagnosed synchronous hormone sensitive oligometastatic prostate cancer.
The STAMPEDE2 trial is a phase III, randomized, open label, multi-center platform trial testing treatments in metastatic hormone sensitive prostate cancer. For the SABR trial, SABR-eligible disease is confirmed on CT/MRI and bone scan and includes:
- 1 to 5 metastatic lesions in bone and/or non-regional nodes
- All lesions are technically suitable for stereotactic ablative body radiotherapy
- Absence of visceral metastases
Key inclusion and exclusion criteria are as follows:
Patients are randomized with 1:1 allocation to arm A (standard of care) or arm S (standard of care + SABR). Standard of care includes long-term ADT, androgen receptor signaling inhibitor, prostate (± pelvic nodes) radiotherapy, ± docetaxel as part of triplet therapy. SABR is given in 3 or 5 fractions, and PSMA PET/CT and/or whole-body MRI imaging are permitted for SABR planning. Treatment decisions based on conventional imaging or next generation imaging will be stratified as follows: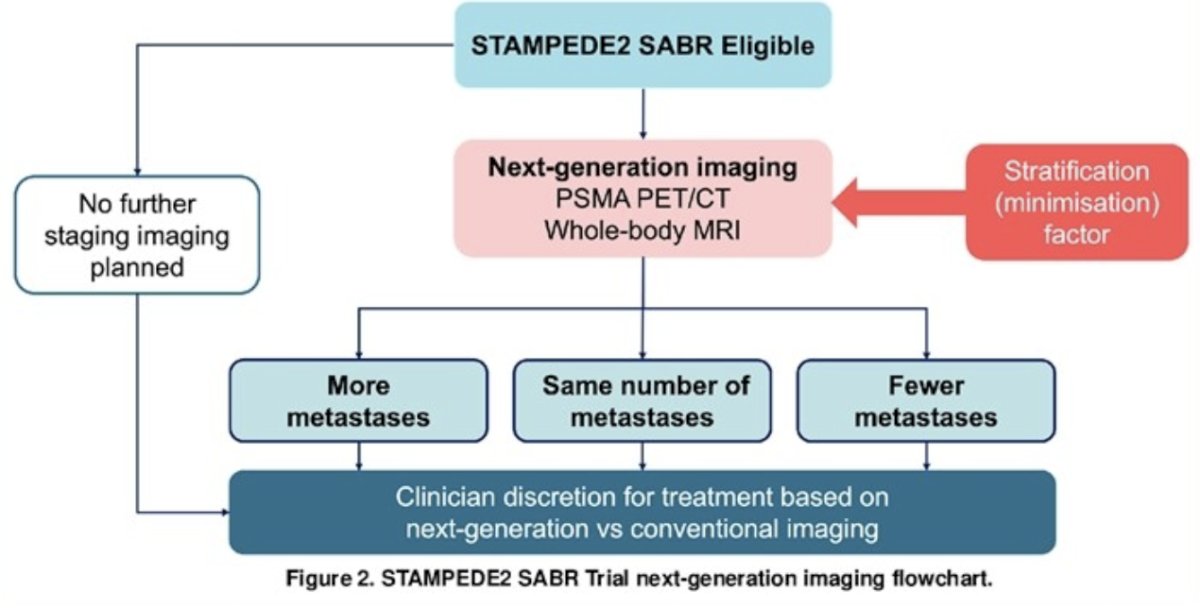
The trial design is as follows: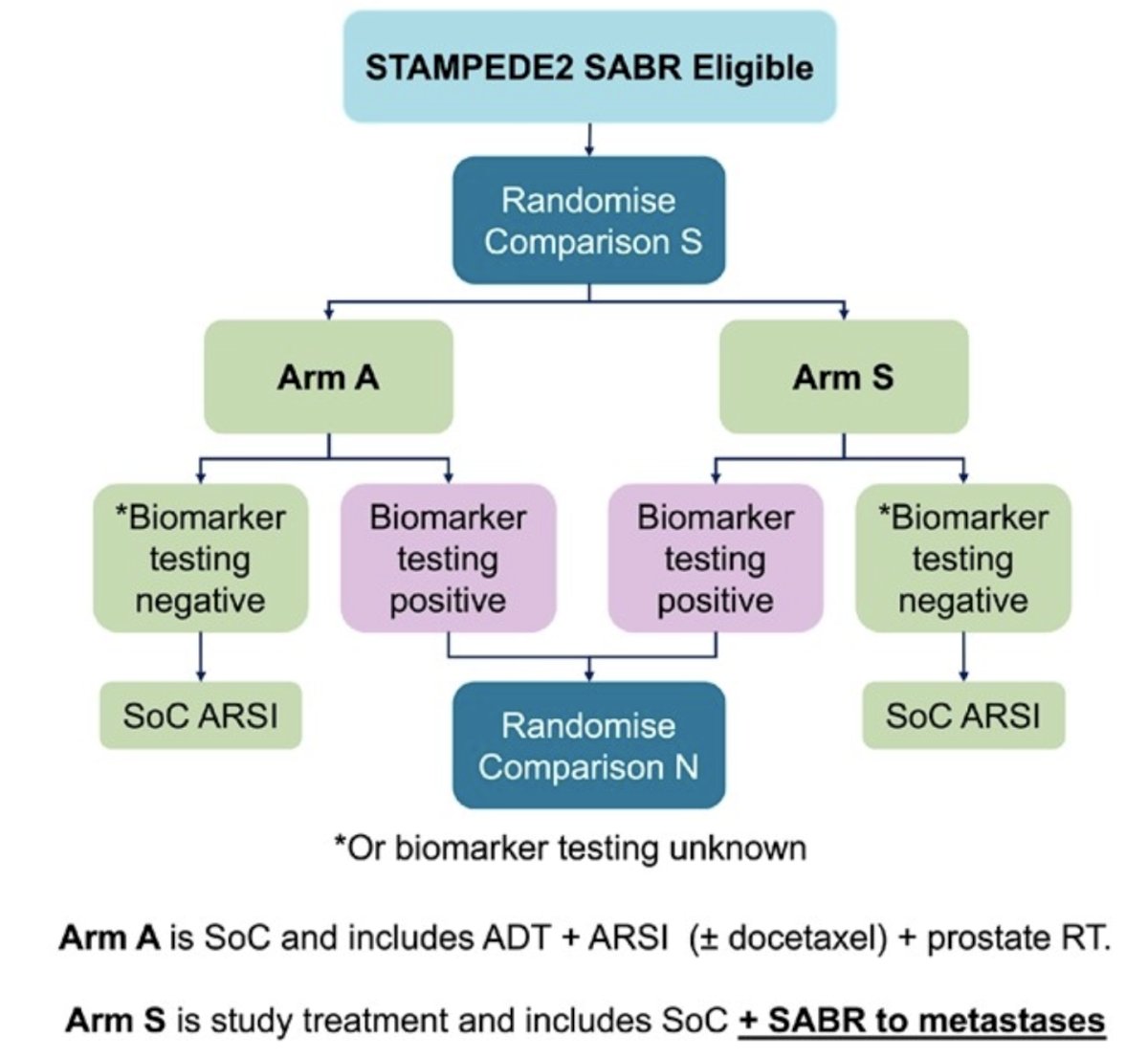
Biomarker testing is offered to all eligible patients. Patients with positive biomarker status are offered a second randomization into the Niraparib arm of the STAMPEDE trial. Target recruitment is 2,476 patients for a target hazard ratio of 0.70 for radiographic progression free survival and 0.75 for overall survival (90% overall pairwise power; co-primary outcomes). A comprehensive radiotherapy quality assurance program is implemented. Secondary outcomes include failure free survival, prostate cancer specific survival, toxicity, quality of life, and cost-effectiveness.
The STAMPEDE2 trial is open to recruitment with the first patient randomized in June 2024. A phased open of the SABR trial is planned in ~60 UK SABR centers. Additionally, a pooled analysis is planned with the METANOVA trial, a spin off the STAMPEDE2 SABR trial protocol. Finally, international expansions in Europe are in progress, and expressions of interest have been received from the USA, India, Nigeria, and New Zealand.
Presented by: Hoda Abdel-Aty, MD, Clinical Research Fellow, Institute of Cancer Research and Royal Marsden Hospital NHS Foundation Trust, London, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: STAMPEDE2 SABR Trial for Oligometastatic Prostate Cancer Treatment - Hoda Abdel-Aty


