(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to the session Biochemical failure post-local therapy: An opportunity for tailored treatment? Dr. Dana Rathkopf discussed the intensification of systemic treatment for poor risk disease.
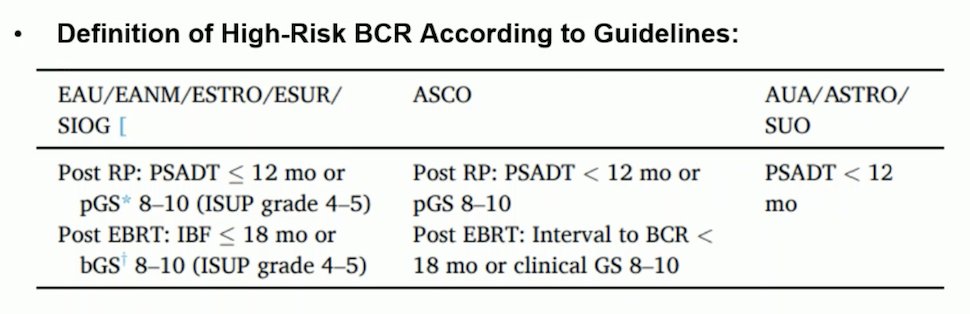
Dr. Rathkopf began by discussing how to define “high-risk” biochemical recurrence in patients with prostate cancer treated with curative intent. There are different definitions and parameters for distinguishing high-risk recurrence versus low-risk, and these variables and definitions continue to evolve. One of the challenges in standardizing the definition is that studies aiming to risk-stratify patients with biochemical recurrence have included very different and heterogeneous populations, making it difficult to establish a single definition. She covered the definitions of high-risk biochemical recurrence according to various guidelines, including the EAU/EANM/ESTRO/ESUR/SIOG guideline, the ASCO guideline, and the AUA/ASTRO/SUO guideline. The different definitions are summarized below:
A major change in the definition of high-risk biochemical recurrence was included in the 2024 NCCN Guidelines. They now recommend the use of multivariable models, such as gene expression classifiers or artificial intelligence (AI)-derived digital histopathology biomarkers. These models combine clinical, pathologic, and other biomarker-derived data to further improve risk stratification.1
Post-prostatectomy biochemical recurrence risk stratification differs from recurrence post-radiation therapy. An ancillary study used radical prostatectomy specimens from the phase 3 placebo-controlled NRG/RTOG 9601 randomized clinical trial. By using the 22-gene Genomic Classifier (GC) score, the study reported that the GC risk group significantly (p=0.003) predicted the risk of distant metastasis and the benefit of adding androgen deprivation therapy (ADT) to early salvage radiation therapy (SRT).2
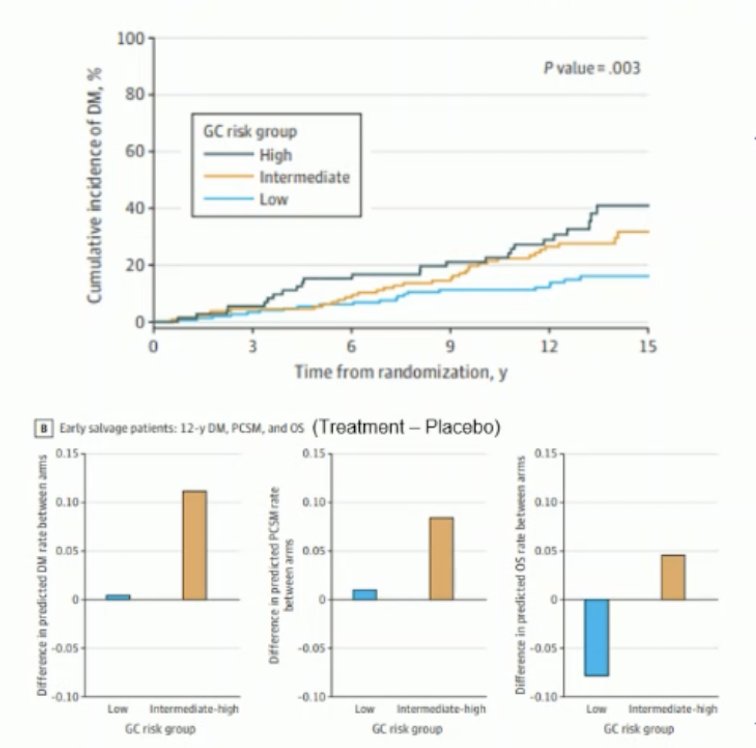
Based on this, the NCCN guidelines recommend that for patients post-radical prostatectomy (RP) with a GC score of ≥0.6, there is a benefit to adding ADT to early salvage radiation therapy (SRT). For patients with a PSA ≤ 0.5 (node-negative) and a low or intermediate GC score, SRT alone may be considered. However, if they have a high-risk GC score, SRT combined with ADT is recommended. The optimal use of GC risk groups in patients receiving late SRT remains unclear.
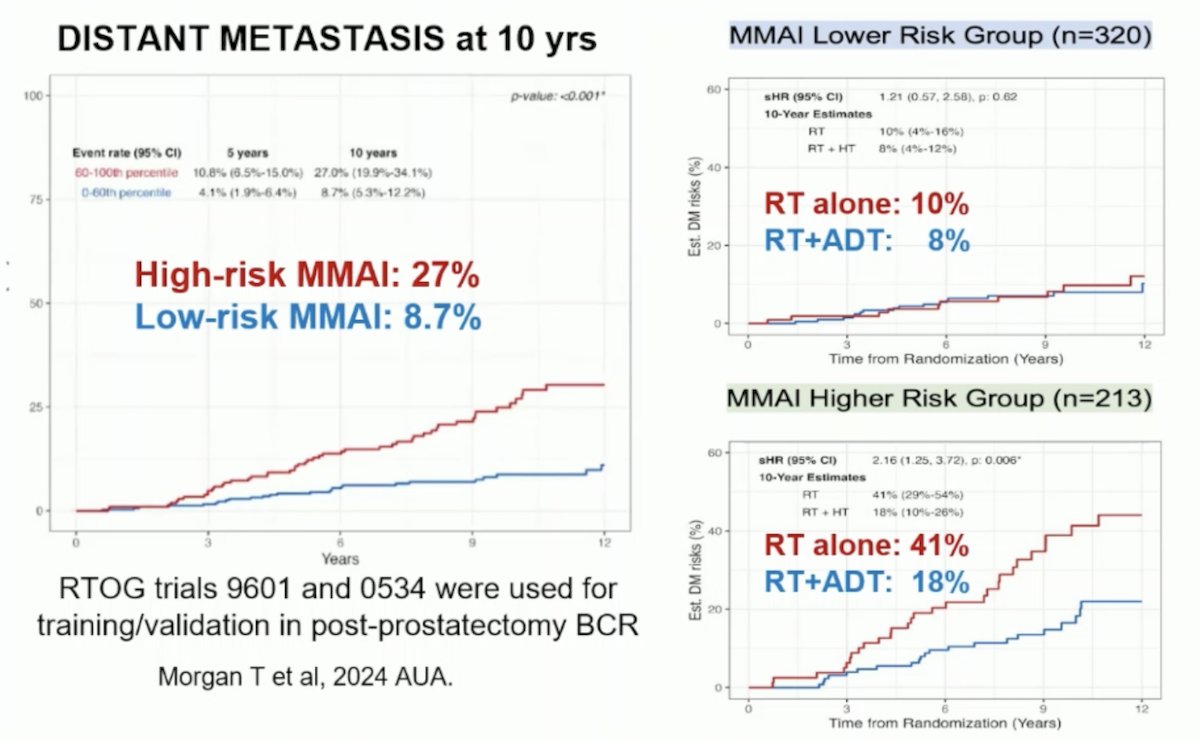
The Multimodal Artificial Intelligence (MMAI)-derived digital pathology-based biomarker is a prognostic tool used for risk stratification post-radical prostatectomy. A study by Todd Morgan and colleagues, presented at AUA this year, utilized data from two phase III randomized trials (NRG/RTOG 9601 and 0534) which enrolled men with biochemical recurrence post-radical prostatectomy. Digitized images of prostatectomy tumor samples were used to develop an MMAI model to predict distant metastasis, incorporating image features with pathologic Grade Group, pathologic T stage, pre-salvage radiotherapy PSA, age, and surgical margin status. The Artera-AI MMAI demonstrated potential as a predictive biomarker by identifying patients most likely to benefit from ADT or SRT alone after biochemical recurrence. The study found that 27% of patients with high-risk MMAI developed metastasis within 10 years compared to 8.7% of those with low-risk MMAI. Among the high-risk group, those who received SRT combined with ADT had a significantly lower risk of developing metastasis.3
She recapitulated the high-risk biochemical recurrence case presented at the beginning of the session. This patient had an ISUP 5, an initial PSA (iPSA) of 10.6 ng/mL, and underwent radical prostatectomy, which revealed a pT3aN0R1 tumor with a rising PSA of 0.6 ng/mL, a PSA doubling time (PSAdt) of less than 6 months, and negative PSMA PET/CT imaging. This patient fulfills the criteria for high-risk biochemical recurrence and, based on current guidelines, would benefit from treatment intensification with ADT and SRT. However, she raised the question of what should be used for systemic treatment intensification.
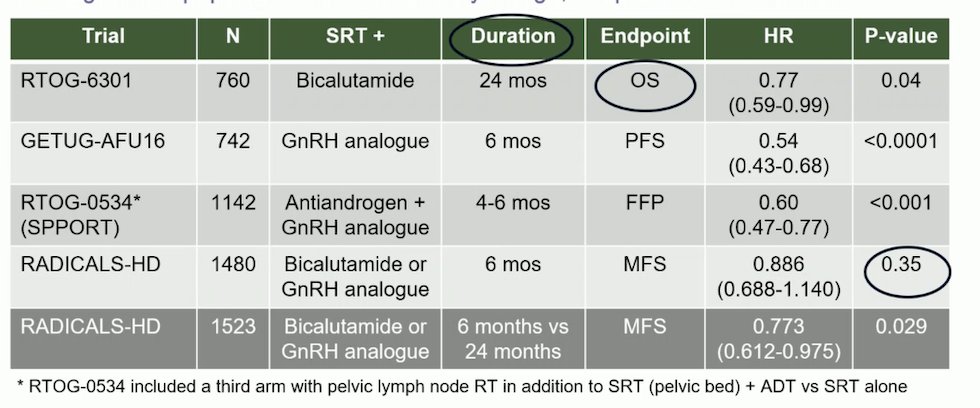
The studies examining SRT with or without the addition of ADT, as well as the duration of ADT, have included heterogeneous populations and varied significantly in design, endpoints, and outcomes. A summary of these studies is presented in the table below. Notably, only one study found a benefit in overall survival: the RTOG-9601 study, which examined bicalutamide for 24 months (HR 0.77, p=0.04). However, another study, RADICALS-HD, examined bicalutamide or a GnRH analogue comparing 6 months of ADT versus no ADT and found no significant difference in terms of metastasis-free survival (p=0.35).4
Looking closer at each of the studies, RTOG-9601, a randomized placebo-controlled phase 3 study, evaluated the addition of bicalutamide for 24 months to SRT in patients with biochemical recurrence after radical prostatectomy. Patients were stratified at study entry based on entry PSA (≥1.5 ng/ml), prior ADT, margin status, and PSA nadir (≥0.5 ng/ml). The primary endpoint was overall survival (OS) with a median follow-up of 13 years. The study found a significant improvement in OS for patients who received bicalutamide for 24 months compared to those who received a placebo. Notably, the greatest OS benefit was seen in patients with a pre-SRT PSA > 1.5 ng/ml.5
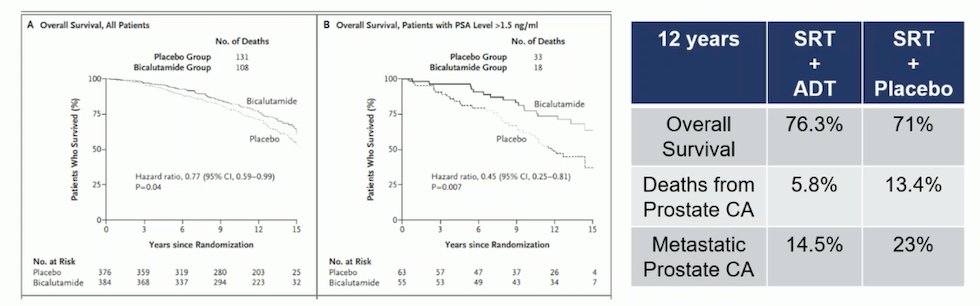
A secondary analysis of the RTOG-9601 study found that in patients with a pre-SRT of ≤ 0.6 there were no clinical benefits with the addition of bicalutamide for 24 months, and there was an increased other cause mortality with ADT.5
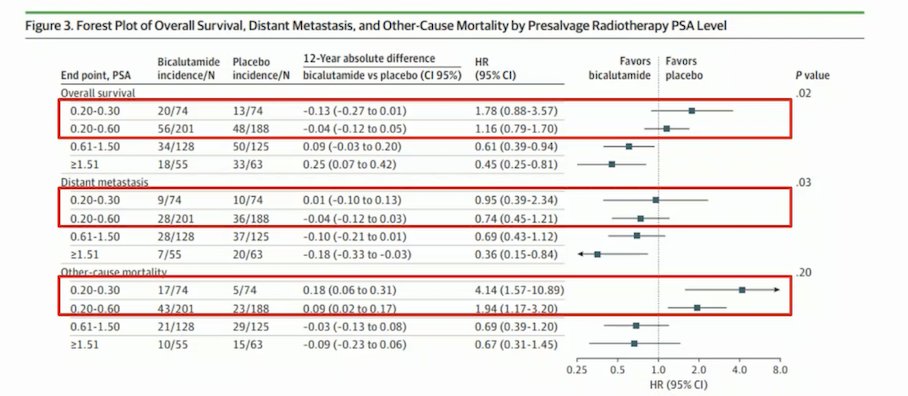
Dr. Rathkopf discussed and compared the GETUG-AFU16 and RTOG-0534 (SPPORT) studies, which examined SRT with or without ADT for 6 months. Notably, the median pre-SRT PSA at entry was <0.5 ng/ml. The primary endpoints included biochemical (PSA) progression-free survival (PFS). The GETUG-AFU16 study met both its primary and secondary endpoints, showing a significant benefit in PSA-PFS (64% vs. 49%) and metastasis-free survival (MFS) (75% vs. 69%) at 10 years. The SPPORT study met its primary endpoint of failure-free progression; however, no significant MFS benefit was observed at 5 years.6,7
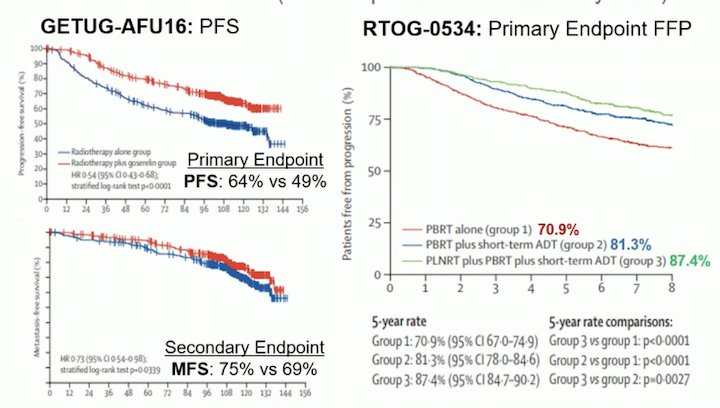
The RADICALS-HD trial is a randomized study that explored the use and duration of ADT with SRT, within the RADICALS protocol, which also addressed questions relating to the timing of radiotherapy (adjuvant versus early salvage). The investigators enrolled men with an indication for SRT who had not received previous post-operative ADT. Following radical prostatectomy but prior to the initiation of SRT, patients were randomized to either no ADT ("None"), 6 months of ADT ("Short"), or 24 months of ADT ("Long"). While 3-way randomization (1:1:1 randomization) was encouraged, 2-way randomization between both None-vs-Short or Short-vs-Long was also allowed.
For this trial, metastasis-free survival (MFS) was chosen as an endpoint based on the ICECAP data, showing MFS is a good surrogate for OS. There was no benefit observed in short ADT versus no ADT. Notably, the short-ADT group had a more favorable population in terms of risk factors (Grade Group, T-stage), and there was a benefit of short-ADT in clinical progression and the need for salvage ADT. The comparison between short-ADT and long-ADT showed a significant improvement in MFS (p=0.029) in favor of long-ADT combined with SRT. However, there was no OS advantage at a median of 9 years because of too few events.4
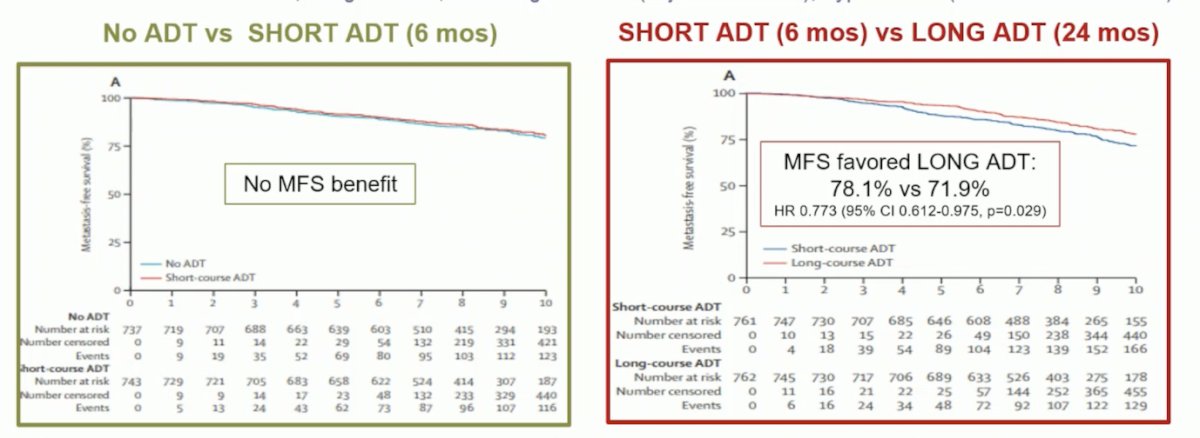
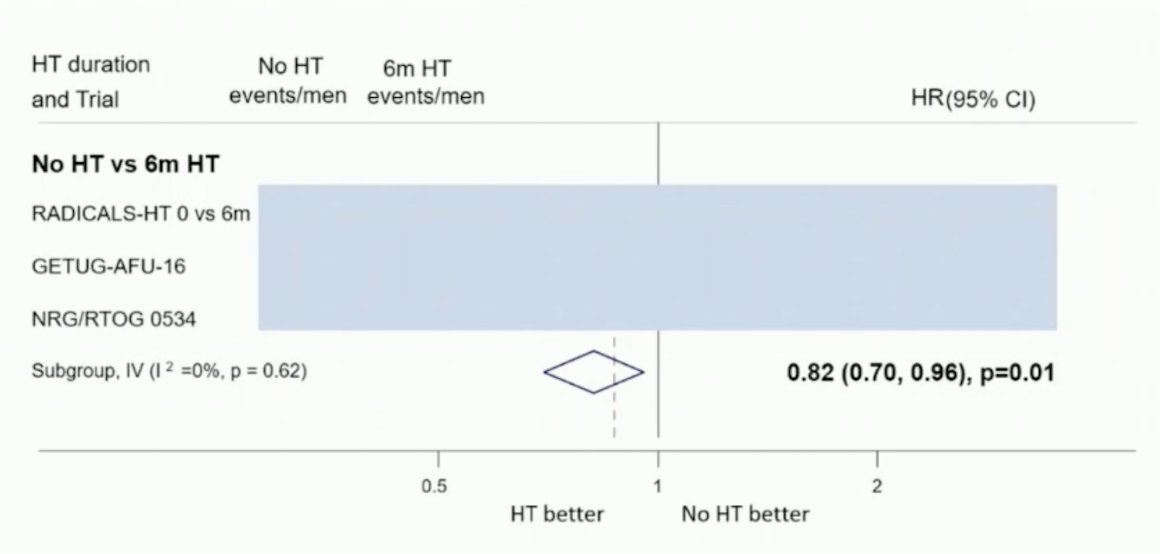
The DASSPORT meta-analysis was a collaborative meta-analysis of aggregate data to assess the duration of ADT with SRT. The collaboration included data from NRG/RTOG 9601, GETUG-AFU 16, NRG/RTOG 0534, and RADICALS-HD. The median follow-up was ≥8 years, and there was no clear improvement in OS with hormone therapy compared to no hormone therapy (HR 0.87, 95% CI 0.75-1.01), irrespective of whether hormone therapy was for 6 months or 24 months. However, based on data from three trials (653 events, 3364 men), there was evidence that 6 months of ADT improved MFS compared to no hormone therapy (HR 0.82, 95% CI 0.70-0.96, p = 0.01), with a 5-year absolute improvement of 2% (CI 0% - 3%).8
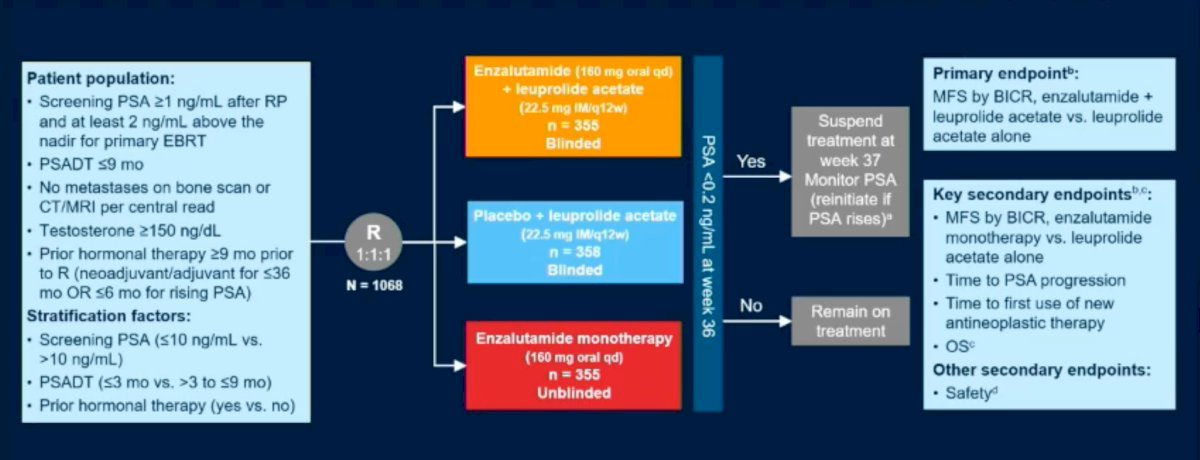
The EMBARK was a three-arm, randomized phase 3 trial that enrolled prostate cancer patients with high-risk biochemical recurrence, defined as a PSA doubling time of ≤9 months and a PSA ≥1 ng/ml after radical prostatectomy or ≥2 ng/ml above nadir following primary EBRT. Patients had no evidence of metastasis on conventional imaging.
Patients were randomly assigned, in a 1:1:1 ratio, to receive one of the following treatments (n=1,068):
- Enzalutamide (160 mg) daily plus ADT
- Placebo plus leuprolide
- Enzalutamide monotherapy
PSA levels were assessed at 36 weeks. If patients had a PSA <0.2, treatment was suspended at week 37, and PSA was monitored with treatment reinitiated if PSA rose again to ≥2 ng/mL (if primary radical prostatectomy) or ≥5 ng/mL (if no primary radical prostatectomy). For those with a PSA >0.2, treatment was continued. The study design is shown below:
The 5-year MFS endpoint was met for enzalutamide plus ADT versus ADT alone (HR 0.42, 95% CI 0.30-0.61) and for enzalutamide monotherapy versus ADT alone (HR 0.63, 95% CI 0.46-0.87). However, OS data remains immature.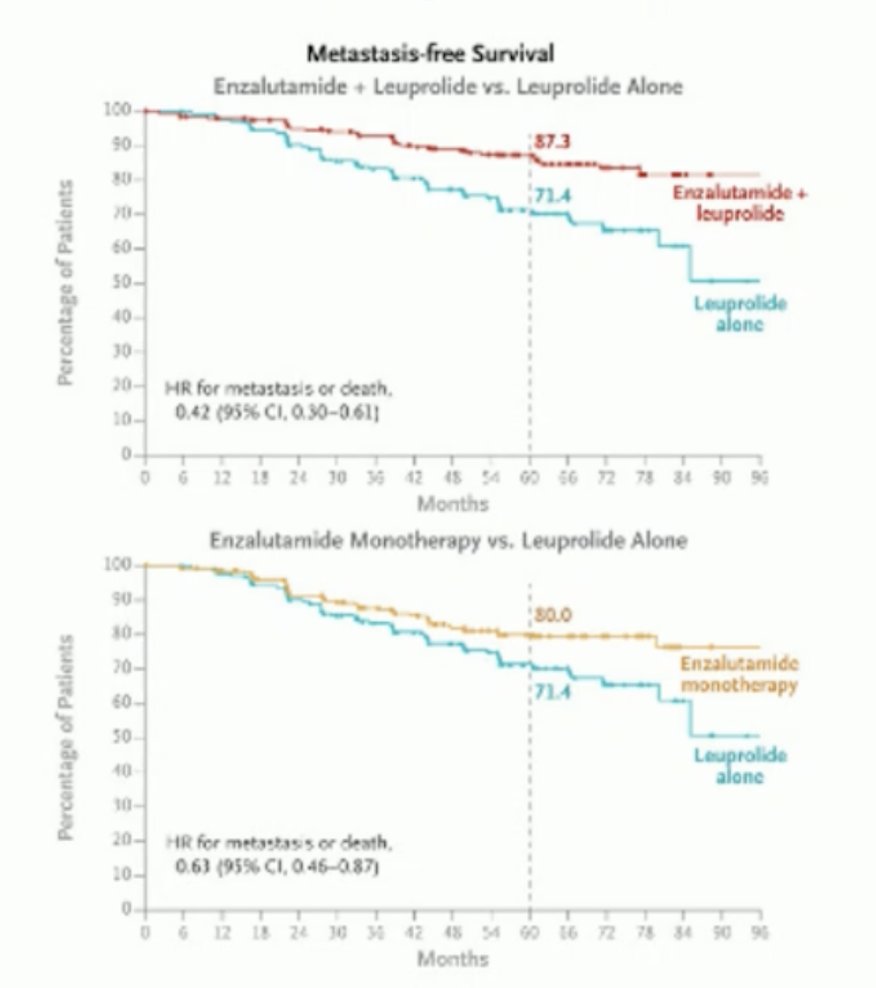
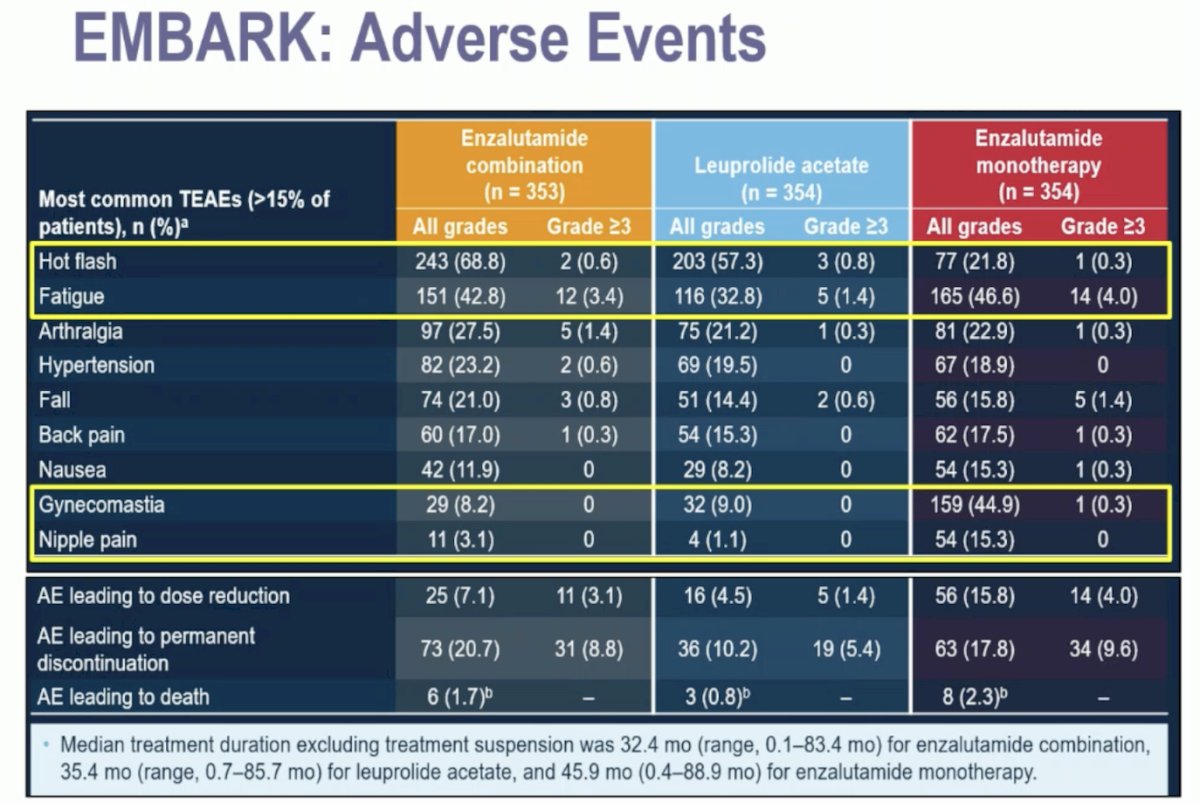
Regarding adverse events, enzalutamide monotherapy was associated with fewer hot flashes. However, despite not receiving ADT, there was no improvement in fatigue, which was the most common reason for discontinuation across all treatment arms. Additionally, there was a high incidence of nipple pain and gynecomastia (44.9%) in the enzalutamide monotherapy group.9
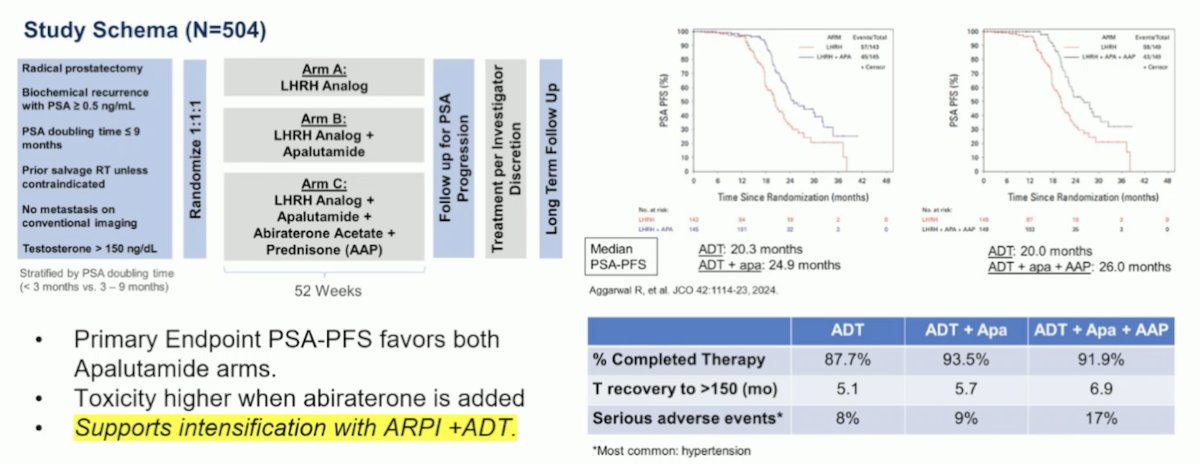
The PRESTO (AFT-19) study is a randomized phase III, open-label trial for patients with biochemically recurrent prostate cancer and a PSA doubling time of ≤9 months (NCT03009981). Patients were randomized 1:1:1 to receive a 52-week treatment course with ADT (control), ADT plus apalutamide, or ADT plus apalutamide and abiraterone. The primary endpoint was PSA progression-free survival (PSA-PFS), defined as serum PSA >0.2 ng/mL after treatment completion. The study's primary endpoint favored both apalutamide arms, although toxicity was higher when abiraterone was added. This data further supports the intensification of treatment with ARPI plus ADT in patients with high-risk biochemically recurrent prostate cancer.10
This data from EMBARK and PRESTO leaves with two options for intensification in high-risk biochemically recurrent prostate cancer (Apalutamide + ADT for 12 months or Enzalutamide +/- ADT for 9 months.
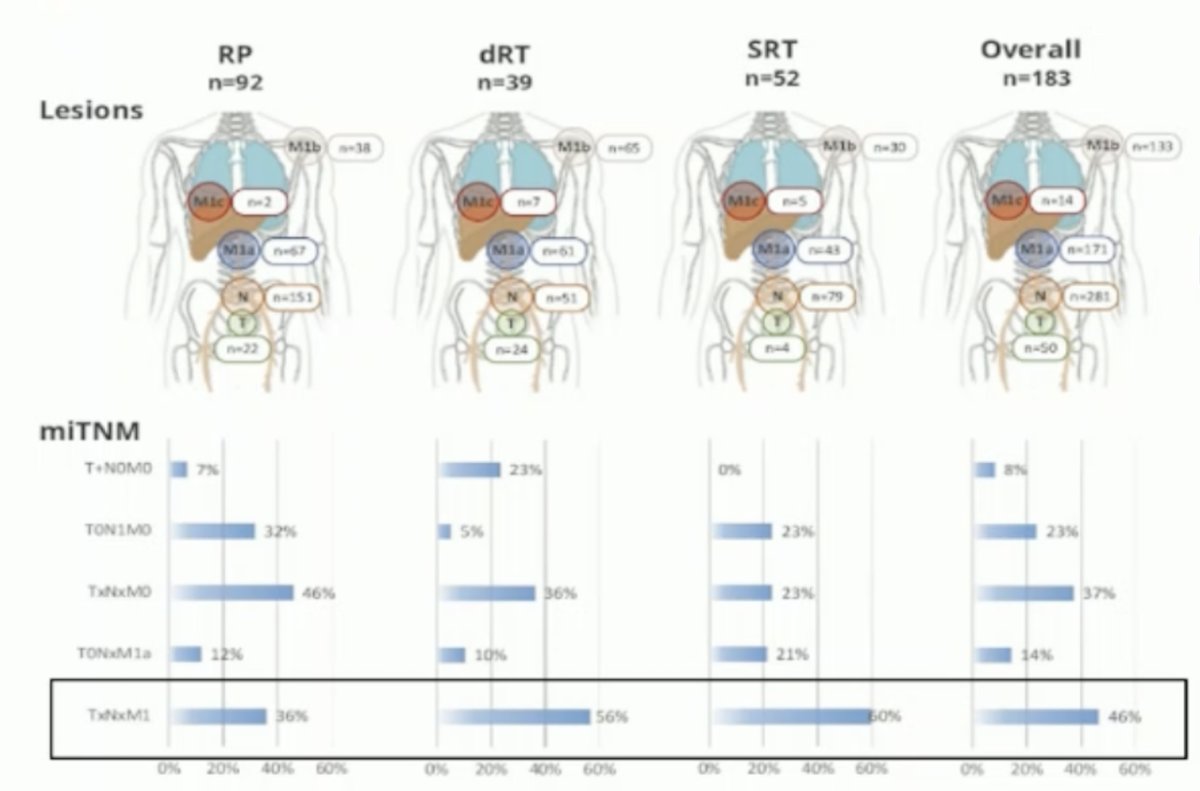
Lastly, data from a study at UCLA, which involved a post-hoc analysis of four prospective studies of PSMA PET using EMBARK eligibility criteria, identified that 46% of patients with EMBARK-like criteria had evidence of distant metastatic disease. This raises the question of whether PSMA-positive BCR patients benefit from accelerating therapy based on a PSMA scan, or if this approach merely adds years of toxicity and its sequelae. Additionally, it suggests that we might already be treating patients with miM1 disease and negative conventional imaging for prostate cancer with treatment intensification using ARPIs.11
To summarize her presentation, Dr. Rathkopf concluded that there is a role for systemic treatment intensification by adding ARPI to ADT in high-risk BCR patients. Enzalutamide monotherapy is an option, but the goals of its use should be carefully considered based on available toxicity and outcome data. Conventional imaging-negative/PSMA PET-positive BCR represents a new treatment paradigm, and prospective studies are ongoing to provide answers to this clinical dilemma.
Presented by: Dana E. Rathkopf, MD, Medical Oncologist, Associate Chair, Junior Faculty Development, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Julian Chavarriaga, MD –Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.4.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed [September 14, 2024]. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Feng FY, Huang HC, Spratt DE, Zhao SG, Sandler HM, Simko JP, Davicioni E, Nguyen PL, Pollack A, Efstathiou JA, Dicker AP, Todorovic T, Margrave J, Liu YS, Dabbas B, Thompson DJS, Das R, Dignam JJ, Sweeney C, Attard G, Bahary JP, Lukka HR, Hall WA, Pisansky TM, Shah AB, Pugh SL, Shipley WU, Tran PT. Validation of a 22-Gene Genomic Classifier in Patients With Recurrent Prostate Cancer: An Ancillary Study of the NRG/RTOG 9601 Randomized Clinical Trial. JAMA Oncol. 2021 Apr 1;7(4):544-552. doi: 10.1001/jamaoncol.2020.7671. Erratum in: JAMA Oncol. 2021 Apr 1;7(4):639. doi: 10.1001/jamaoncol.2021.0552. PMID: 33570548; PMCID: PMC7879385.
- Morgan TM, Ren Y, Tang S, Zwerink W, Chen E, Mitani A, et al. PD42-11 DEVELOPMENT AND VALIDATION OF A MULTIMODAL ARTIFICIAL INTELLIGENCE (MMAI)-DERIVED DIGITAL PATHOLOGY-BASED BIOMARKER PREDICTING METASTASIS FOR RADICAL PROSTATECTOMY PATIENTS WITH BIOCHEMICAL RECURRENCE IN NRG/RTOG TRIALS. Journal of Urology [Internet]. 2024 May 1 [cited 2024 Sep 14];211(5S):e897. Available from: https://doi.org/10.1097/01.JU.0001008560.54103.65.11
- Parker CC, Kynaston H, Cook AD, Clarke NW, Catton CN, Cross WR, Petersen PM, Persad RA, Pugh CA, Saad F, Logue J, Payne H, Bower LC, Brawley C, Rauchenberger M, Barkati M, Bottomley DM, Brasso K, Chung HT, Chung PWM, Conroy R, Falconer A, Ford V, Goh CL, Heath CM, James ND, Kim-Sing C, Kodavatiganti R, Malone SC, Morris SL, Nabid A, Ong AD, Raman R, Rodda S, Wells P, Worlding J, Parulekar WR, Parmar MKB, Sydes MR; RADICALS investigators. Duration of androgen deprivation therapy with postoperative radiotherapy for prostate cancer: a comparison of long-course versus short-course androgen deprivation therapy in the RADICALS-HD randomised trial. Lancet. 2024 Jun 1;403(10442):2416-2425. doi: 10.1016/S0140-6736(24)00549-X. Epub 2024 May 16. PMID: 38763153; PMCID: PMC7616389.
- Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, Sartor O, Patel MP, Bahary JP, Zietman AL, Pisansky TM, Zeitzer KL, Lawton CA, Feng FY, Lovett RD, Balogh AG, Souhami L, Rosenthal SA, Kerlin KJ, Dignam JJ, Pugh SL, Sandler HM; NRG Oncology RTOG. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med. 2017 Feb 2;376(5):417-428. doi: 10.1056/NEJMoa1607529. PMID: 28146658; PMCID: PMC5444881.
- Pollack A, Karrison TG, Balogh AG, Gomella LG, Low DA, Bruner DW, Wefel JS, Martin AG, Michalski JM, Angyalfi SJ, Lukka H, Faria SL, Rodrigues GB, Beauchemin MC, Lee RJ, Seaward SA, Allen AM, Monitto DC, Seiferheld W, Sartor O, Feng F, Sandler HM. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial. Lancet. 2022 May 14;399(10338):1886-1901. doi: 10.1016/S0140-6736(21)01790-6. PMID: 35569466; PMCID: PMC9819649.
- Carrie C, Hasbini A, de Laroche G, Richaud P, Guerif S, Latorzeff I, Supiot S, Bosset M, Lagrange JL, Beckendorf V, Lesaunier F, Dubray B, Wagner JP, N'Guyen TD, Suchaud JP, Créhange G, Barbier N, Habibian M, Ferlay C, Fourneret P, Ruffion A, Dussart S. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016 Jun;17(6):747-756. doi: 10.1016/S1470-2045(16)00111-X. Epub 2016 May 6. Erratum in: Lancet Oncol. 2016 Jun;17(6):e223. doi: 10.1016/S1470-2045(16)30170-X. Erratum in: Lancet Oncol. 2019 Jun;20(6):e293. doi: 10.1016/S1470-2045(19)30347-X. PMID: 27160475.
- Burdett S et al. 2022 ESMO Annual Meeting, LBA64
- Freedland SJ, de Almeida Luz M, De Giorgi U, Gleave M, Gotto GT, Pieczonka CM, Haas GP, Kim CS, Ramirez-Backhaus M, Rannikko A, Tarazi J, Sridharan S, Sugg J, Tang Y, Tutrone RF Jr, Venugopal B, Villers A, Woo HH, Zohren F, Shore ND. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med. 2023 Oct 19;389(16):1453-1465. doi: 10.1056/NEJMoa2303974. PMID: 37851874.
- Aggarwal R, Heller G, Hillman DW, Xiao H, Picus J, Taplin ME, Dorff T, Appleman L, Weckstein D, Patnaik A, Bryce A, Shevrin D, Mohler J, Anderson D, Rao A, Tagawa S, Tan A, Halabi S, Dooley K, O'Brien P, Chen R, Ryan CJ, Eggener SE, Morris MJ; EORTC-55994 Study Group. PRESTO: A Phase III, Open-Label Study of Intensification of Androgen Blockade in Patients With High-Risk Biochemically Relapsed Castration-Sensitive Prostate Cancer (AFT-19). J Clin Oncol. 2024 Apr 1;42(10):1114-1123. doi: 10.1200/JCO.23.01157. Epub 2024 Jan 23. PMID: 38261983.
- Armstrong W et al. 2023 ASCO Annual Meeting, Abstract 5091


