(UroToday.com) The 2024 ESMO annual meeting included a session highlighting prostate cancer trials in progress, with Dr. Minal Padden-Modi discussing the trial design of STAMPEDE2, a phase 3, randomized, open-label trial of 177Lu-PSMA-617 in patients with newly diagnosed metastatic prostate cancer starting ADT. The rationale behind using an accelerated schedule of 177Lu-PSMA-617 is because ADT and androgen receptor pathway inhibitors can upregulate PSMA expression in the short term. The schedule also aims to leverage rapid responses expected in hormone sensitive disease to hormone therapy. 177Lu-PSMA-617 is a ß-emitting radioligand therapy with radiographic progression-free survival and overall survival benefit in castrate-resistant prostate cancer following taxane chemotherapy.1 The trial tests an upfront accelerated dose schedule for 177Lu-PSMA-617, in addition to standard of care.
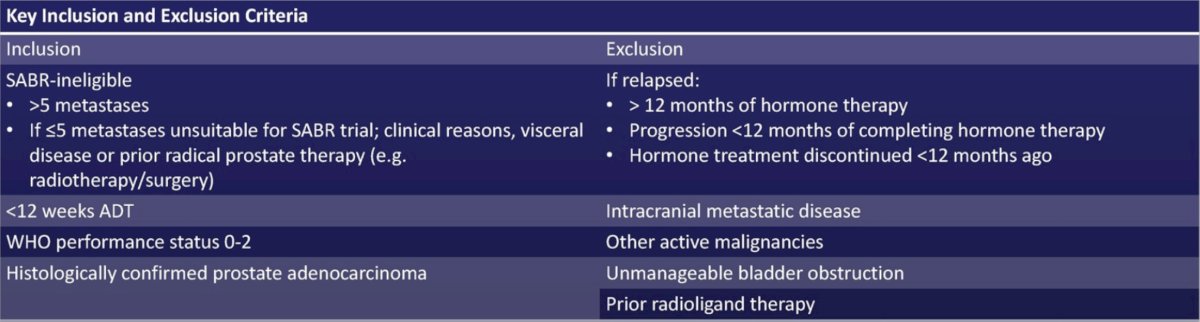
STAMPEDE2 is a phase III, randomized, open label, multi-center platform protocol testing treatments in patients with metastatic prostate cancer starting ADT (NCT06320067, ISRCTN66357938). For the 177Lu-PSMA-617 trial, eligibility is confirmed on conventional imaging with CT/MRI and bone scan. Full inclusion and exclusion criteria are as follows:
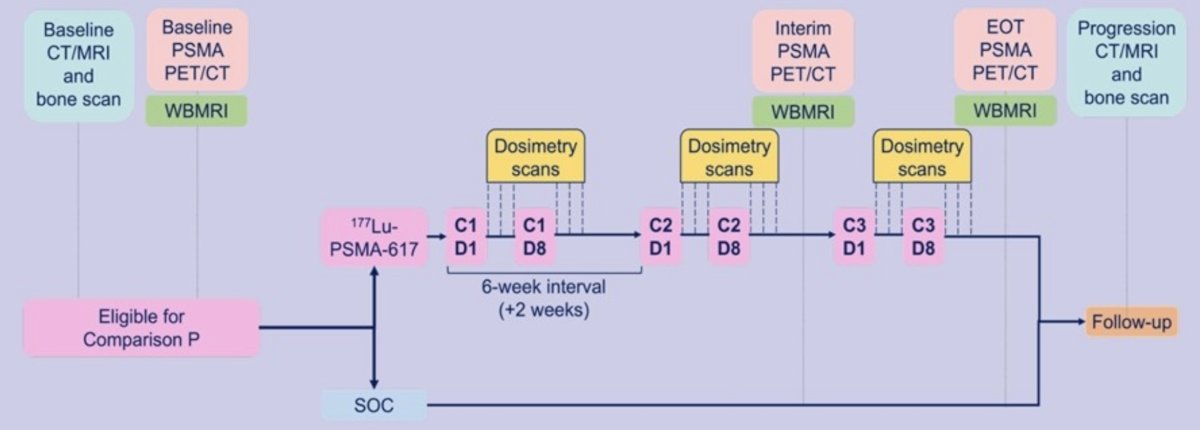
Patients are randomized (1:1 allocation) to arm A (standard of care) or arm P (standard of care + 177Lu-PSMA-617). Standard of care includes long-term ADT, androgen receptor signaling inhibitor, ± prostate radiotherapy, ± docetaxel as part of ‘triplet therapy’. 177Lu-PSMA-617 is given at 7.4 GBq on day 1 and day 8, 3x 6-weekly cycles. The trial design is as follows:

An embedded imaging sub-study will evaluate 177Lu-PSMA-617 treatment response and dose uptake by tumor and normal tissue in ~40-100 men. The imaging sub-study includes paired PSMA PET/CT and whole-body MRI scans at baseline, after 2 cycles, and end of 177Lu-PSMA-617 treatment as well as three time point dosimetry studies per 177Lu-PSMA-617 dose:
Biomarker testing is offered to all eligible patients. Patients with positive biomarker status are offered a second randomization into the STAMPEDE2 niraparib trial arm. Target recruitment is 1,756 patients with dual primary outcomes of radiographic progression-free survival and overall survival. Secondary endpoints include failure free survival, prostate cancer specific survival, quality of life, safety, toxicity, compliance, and cost-effectiveness. The target hazard ratio is 0.70 for radiographic progression-free survival and 0.75 for overall survival (90% overall pairwise power). The expected reporting time for radiographic progression free survival is ~42 months and for overall survival is ~62 months.
Accrual has commenced in the UK with the first patient in June 2024. The trial is planned to be conducted in 20 177Lu-PSMA-617 delivering sites in the UK. International expansion is anticipated with wave 1 sites opening in 2025 in EU member states. Further international participation is expected in wave 2.
Presented by: Minal Padden-Modi, Institute of Cancer Research and Royal Marsden Hospital NHS Foundation Trust, London, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: Accelerated 177Lu-PSMA-617 Protocol Tested in STAMPEDE2 - Minal Padden-Modi & Nicholas James


