The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a genitourinary cancers poster session. Dr. Dana Rathkopf presented the results of SAABR, a single arm phase II trial of combination androgen receptor pathway inhibitor (ARPI), a GnRH analog, and atezolizumab.
Androgen deprivation therapy (ADT) combined with an androgen receptor pathway inhibitor (ARPI) plus prostate-directed radiation therapy is considered standard of care for men with low-volume metastatic hormone-sensitive prostate cancer (mHSPC).1 Stereotactic body radiotherapy (SBRT) and ADT each profoundly remodel the immune microenvironment, which includes increased expression of PD-L1 on tumor and immune cells. The study investigators hypothesized that adding atezolizumab (anti-PD-L1 therapy) in combination with androgen ablation and SBRT could result in durable anti-tumor responses in newly diagnosed metastatic prostate cancer.
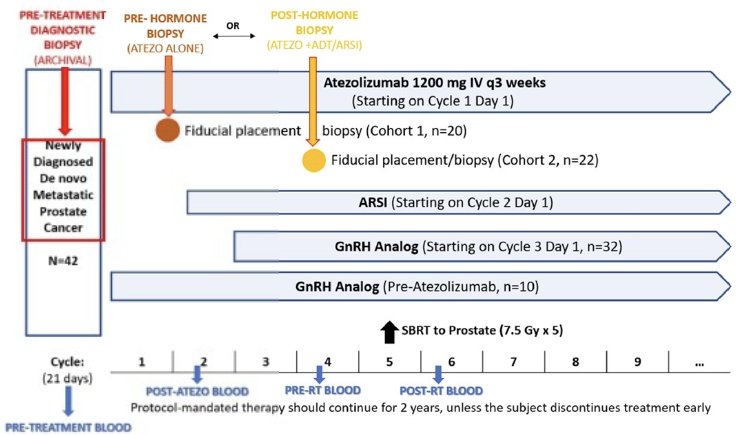
SAABR is a single arm, phase II trial of atezolizumab (1,200mg IV every 3 weeks) + an ARPI (abiraterone 1,000mg + prednisone 5mg once daily or enzalutamide 160mg once daily) + a GnRH analog + SBRT to the prostate (7.5 Gy x 5 fractions) in mHSPC patients. Cohort 1 subjects were treatment-naïve at Cycle 1 Day 1. Cohort 2 subjects were on ADT prior to enrollment.
The trial eligibility criteria were as follows:
- Newly diagnosed untreated mHSPC
- Eligible for SBRT
- No prior definitive treatment for prostate cancer
- 10 patients on ADT up to 90 days prior to enrollment were eligible if they demonstrated a PSA decline
The target sample size was 42 subjects that received ≥1 dose of atezolizumab. The primary endpoint was two-years freedom-from-failure at two years, relative to the historical rate in the STAMPEDE trial (70% for the metastatic cohort). Failure was defined as biochemical failure, radiographic progression per PCWG3 criteria, or death due to any cause. Key secondary endpoints included:
- Safety and tolerability using CTCAE v5.0
- Prostate cancer-specific survival
- Overall survival
Correlative endpoints included:
- Immunogenic predictors of treatment response
- The effects of anti-PD-L1 alone or combined with hormonal therapy on the prostate tumor microenvironment
- To determine whether therapy induces a systemic anti-tumor response
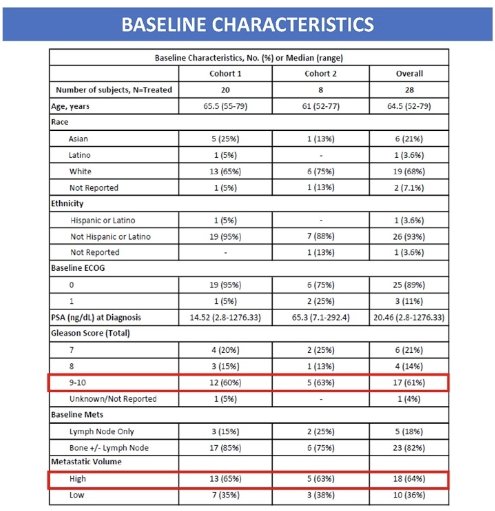
The baseline patient characteristics are summarized below. Twenty-eight patients were enrolled (Cohort 1: 20; Cohort 2: 8). The median patient age was 64.5 years. The median PSA at diagnosis was 20.5 ng/ml. 61% of patients had Gleason Score 9–10 at baseline. 64% of patients had high volume metastatic disease at baseline.
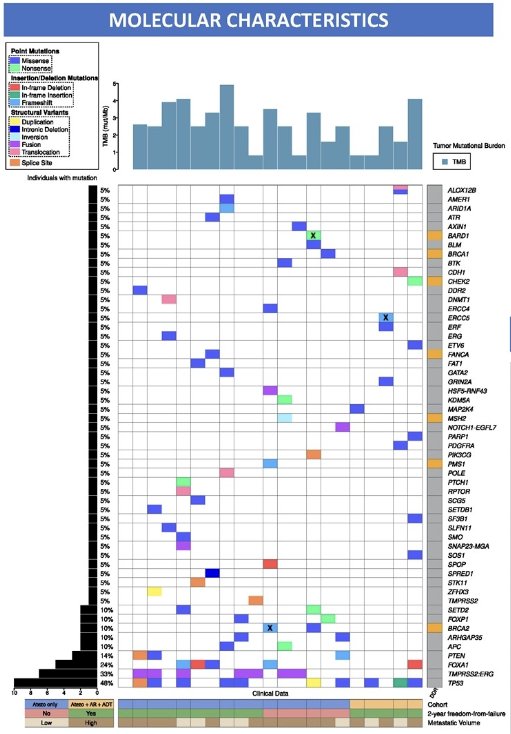
From a molecular standpoint, 48% of patients had an aggressive phenotype (TP53, PTEN mutations). 25% of patients had homologous recombination repair (HRR) mutations (germline: 15%).
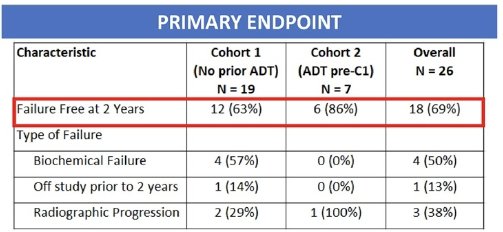
Overall, 69% of patients were free of failure at two years (Cohort 1: 63%, Cohort 2: 86%). There was no significant difference between the observed failure rate at two years in this cohort and that observed in historical series (70%).
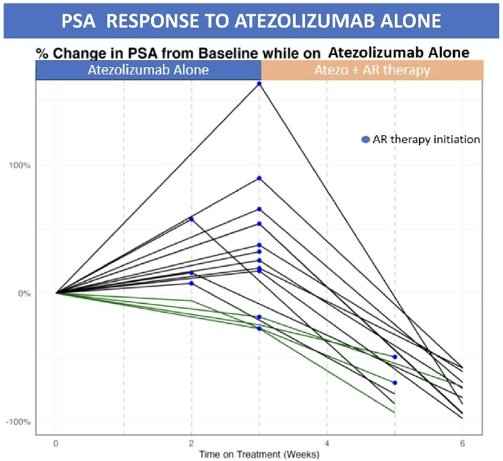
4/20 (20%) patients in this group had a PSA response on atezolizumab alone. All 4 patients were from Cohort 1 (no prior ADT at trial entry).
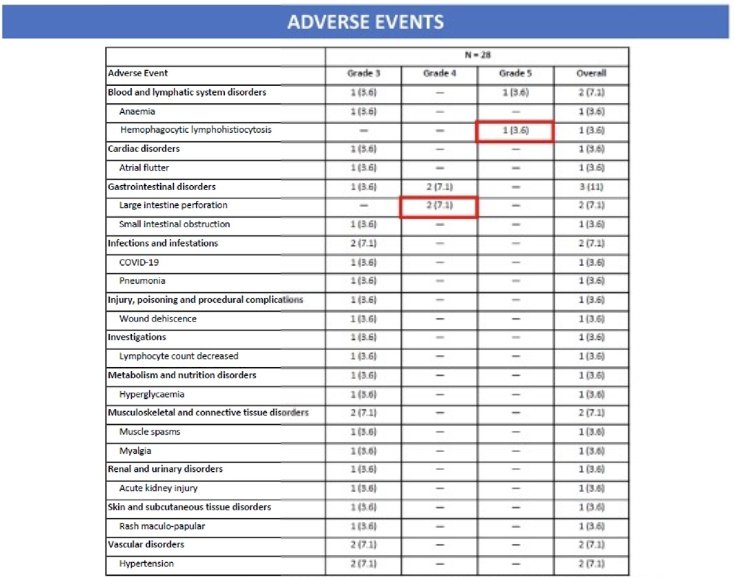
Two patients had a grade 4 colonic perforation on study within two months of SBRT. One patient died after a single treatment with atezolizumab from hemophagocytic lymphocytosis (without SBRT or hormone therapy). Due to excessive toxicity, study enrollment was discontinued.
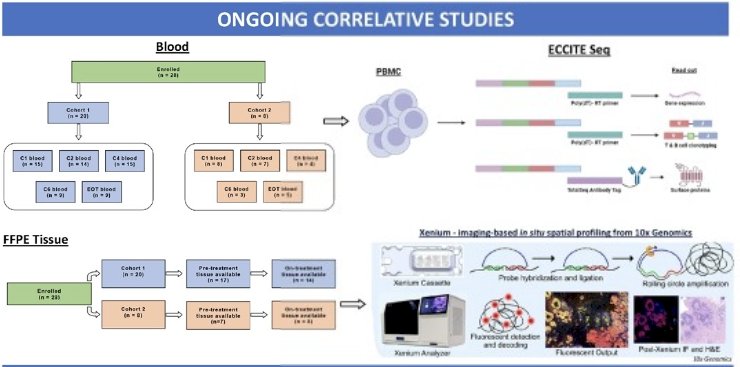
Correlative studies are ongoing to identify potential biomarkers of response:
Dr. Rathkopf concluded as follows:
- Maximal hormonal blockade (ADT + ARPI) combined with atezolizumab plus SBRT is associated with significant toxicity and modest activity
- A subset of patients demonstrates PSA response to atezolizumab alone
- Additional correlative studies are aimed at identifying potential biomarkers of response
Presented by: Dr. Dana E. Rathkopf, MD, Genitourinary Oncologist, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:- Bossi A, Foulon S, Maldonado X, et al. Prostate irradiation in men with de novo, low-volume, metastatic, castration-sensitive prostate cancer (mCSPC): Results of PEACE-1, a phase 3 randomized trial with a 2x2 design. J Clin Oncol. 2023; 41:Number 17_suppl.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomized controlled phase 3 trial. Lancet. 2018; 392(10162):2353-66.
- Boeve L, Hulshof MCCM, Verhagen PCMS, et al. Patient-reported quality of life in patients with primary metastatic prostate cancer treated with androgen deprivation therapy with and without concurrent radiation therapy to the prostate in a prospective randomized clinical trial: Data from the HORRAD trial. Eur Urol. 2021;79(2) :188-97.


