(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to the session Biochemical failure post-local therapy: An opportunity for tailored treatment? Dr. Derya Tilki discussed surveillance or minimal intervention for patients with good risk prostate cancer.
This session began with a case presentation by Dr. Pierre Blanchard. He presented two cases of patients with biochemical recurrence (BCR) after radical prostatectomy:
- The first case involved a patient with high-risk BCR. This patient had a prostate cancer ISUP grade group 5, an initial PSA (iPSA) of 10.6 ng/mL, and underwent radical prostatectomy, which revealed a pT3aN0R1 (positive surgical margin, 2 mm). After treatment, the patient experienced a rising PSA of 0.6 ng/mL, a PSA doubling time (PSAdt) of less than 6 months, and negative PSMA PET/CT imaging.
- The second case was of a 70-year-old patient, 7 years post-radical prostatectomy, with a Gleason grade group 2 and negative surgical margins (pT2N0M0). This patient had a rising PSA of 0.08, 0.16, and 0.23 ng/mL, with negative PSMA PET/CT and a GC score of 0.30 (Decipher low-risk group), indicating a patient with low-risk BCR.
Dr. Tilki began by highlighting the importance of risk stratification in patients with BCR to inform treatment decision-making. A recent systematic review assessed risk stratification of patients with recurrence after primary treatment for prostate cancer. This review included 37 studies (n=10,632), with 25 focusing on post-prostatectomy patients (n=9,010) and 12 on post-radiotherapy patients (n=1,622). In patients with BCR post-prostatectomy, factors associated with adverse outcomes include higher pathological T stage, grade group, and negative surgical margins (≥pT3, GG ≥4, and R0), PSA doubling time (PSADT) < 6 months, higher PSA prior to salvage treatment, shorter time to recurrence, the 22-gene tumor RNA signature (Decipher), and recurrence location on molecular imaging. In patients with BCR after radiotherapy, factors associated with adverse outcomes include a shorter time to recurrence, PSADT < 6 months, and higher PSA velocity.1
She highlighted the NCCN 2024 guideline for prostate cancer and its risk stratification for patients with BCR post-radical prostatectomy with node-negative disease planned for salvage radiotherapy (SRT).2 The guideline recommends using Decipher, a 22-gene genomic classifier assay. Based on the genomic classifier (GC) score, patients will be classified as high-risk BCR or low-risk BCR, with the threshold being <0.6 versus ≥0.60 and those with high-risk BCR will benefit from SRT + androgen deprivation therapy (ADT).
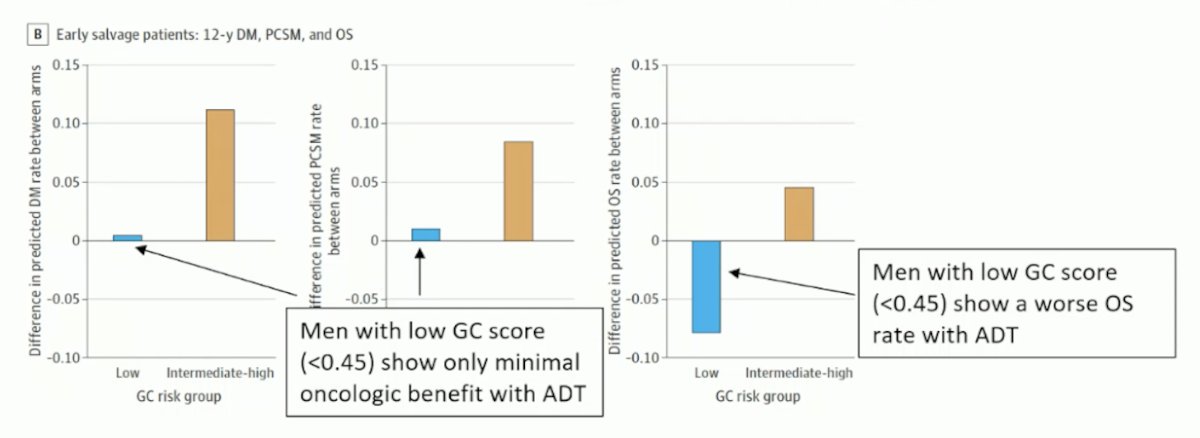
This recommendation is based on two phase III randomized trials post-radical prostatectomy (RP): an ancillary analysis of the NRG/RTOG 9601.3 Using Decipher or the genomic classifier (GC) score to inform SRT with or without ADT, this study demonstrated the independent prognostic effect of the GC on prostate cancer-specific mortality and overall survival (OS). Men with a low GC score (<0.45) show only minimal oncologic benefit with adding ADT to SRT; notably, men with a low GC score (<0.45) show a worse OS rate when ADT was added to SRT. This reinforces that not all men with BCR benefit from adding ADT to SRT.
The second trial was the SAKK 09/10 phase III trial, which tested post-RP lower versus higher dose RT alone.4 The study demonstrated the independent prognostic effect of GC on biochemical progression, clinical progression, secondary hormone therapy, and metastasis-free survival (MFS).
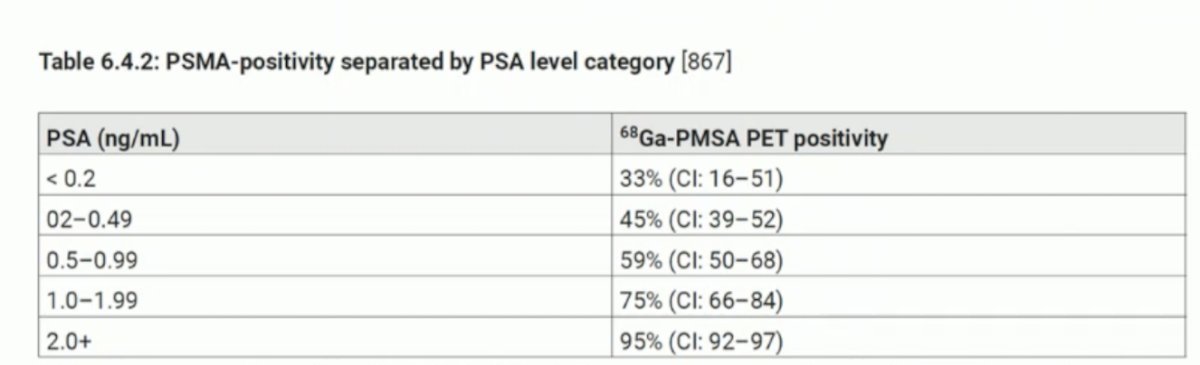
Imaging for biochemical recurrenceIn patients with biochemical recurrence (BCR), imaging can help detect both local recurrences and distant metastases. Conventional imaging requires a high PSA level to increase its sensitivity. After radical prostatectomy (RP), PSMA PET/CT is the imaging modality with the highest sensitivity at low PSA levels (< 0.5 ng/mL). However, the sensitivity of PSMA PET/CT detection still depends on the PSA level. She presented the rate of PSMA positivity reported by the EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer, which for patients with PSA between 0.2 and 0.49 was 45%.
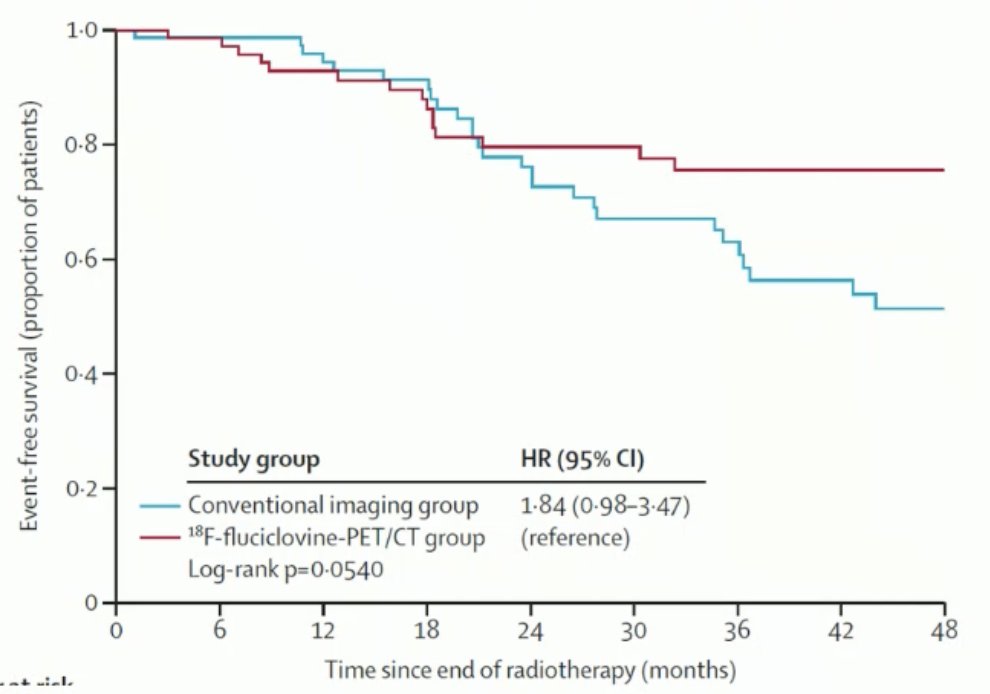
The EMPIRE-1 study was a single-center, open-label phase 2/3 randomized controlled trial exploring 18F-Fluciclovine PET/CT imaging versus conventional imaging to guide salvage radiotherapy (SRT) in patients with biochemical recurrence (BCR) post-radical prostatectomy. This study enrolled 165 patients who were randomized. They compared the three-year failure-free survival rate for patients who had conventional imaging versus those who had 18F-Fluciclovine PET/CT. The event-free survival at 4 years was 51.2% versus 75.5% (p < 0.0001) in favor of 18F-Fluciclovine PET/CT. Inclusion of 18F-Fluciclovine PET into post-prostatectomy radiotherapy planning significantly improved event-free survival.5
Dr. Tilki emphasized the importance of risk stratification in patients with biochemical recurrence (BCR) to inform treatment decision-making. The EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer recommend offering monitoring (PSA) to patients with EAU low-risk BCR. The European Association of Urology (EAU) BCR risk stratification system, proposed by the EAU prostate cancer guideline update, defines low-risk BCR after radical prostatectomy (RP) as patients with a PSA doubling time (PSADT) > 12 months and a Gleason score < 8. High-risk BCR after RP is defined as patients with a PSADT ≤ 12 months or a Gleason score of at least 8.6 The potential benefits and toxicities of salvage treatment should be discussed with each individual patient, considering both the EAU BCR risk stratification and life expectancy and avoid overtreatment for the low-risk BCR group.
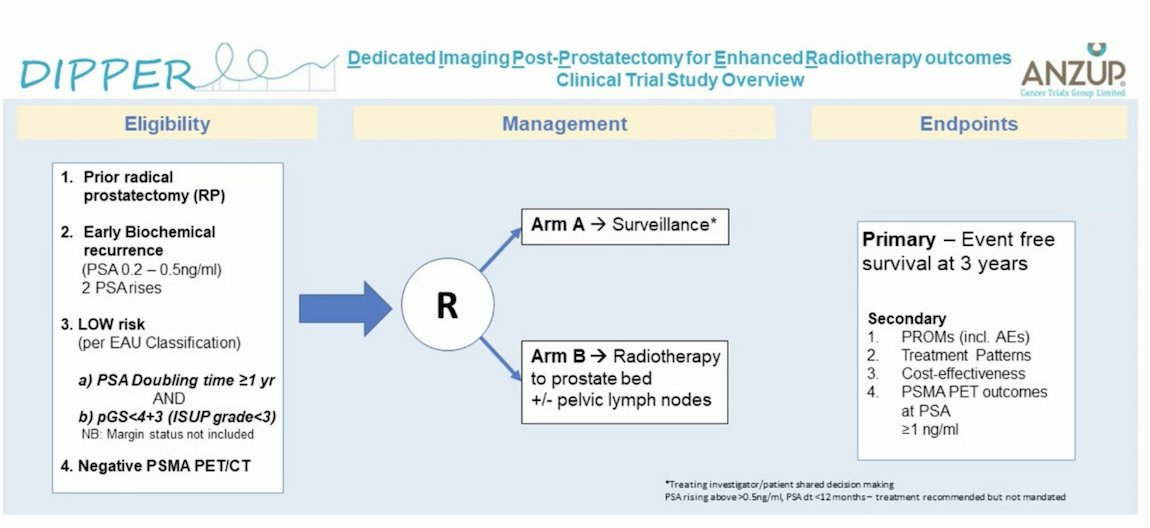
The DIPPER (Dedicated Imaging Post-Prostatectomy for Enhanced Radiotherapy Outcomes) Clinical Trial Study, led by ANZUP in Australia, targets patients with prior radical prostatectomy and early biochemical recurrence (PSA 0.2-0.5 ng/ml) confirmed on two PSA rises. Eligible participants are those classified as low-risk BCR per the EAU risk classification and have a negative PSMA PET/CT. This study is randomizing patients with low-risk BCR to either surveillance or salvage radiotherapy (SRT). The trial aims to recruit 100 patients and is still enrolling participants.7
A study led by Dr. Tilki assessed PSA levels at the time of salvage radiotherapy (SRT) after radical prostatectomy using a multinational database of 25,551 patients with pT2-4N0 or NXM0 prostate cancer. Multivariable Cox regression analysis was used to evaluate whether there was a significant increase in cancer mortality risk when SRT was delivered above a prespecified PSA level starting at 0.10 ng/mL. The study found that among patients with at most one high-risk factor (i.e., pT3/4 or prostatectomy Gleason score 8-10), those who received SRT at a PSA level >0.25 ng/mL had a significantly higher prostate cancer mortality compared to men who received SRT when the PSA was ≤0.25 ng/mL (p = 0.008).8
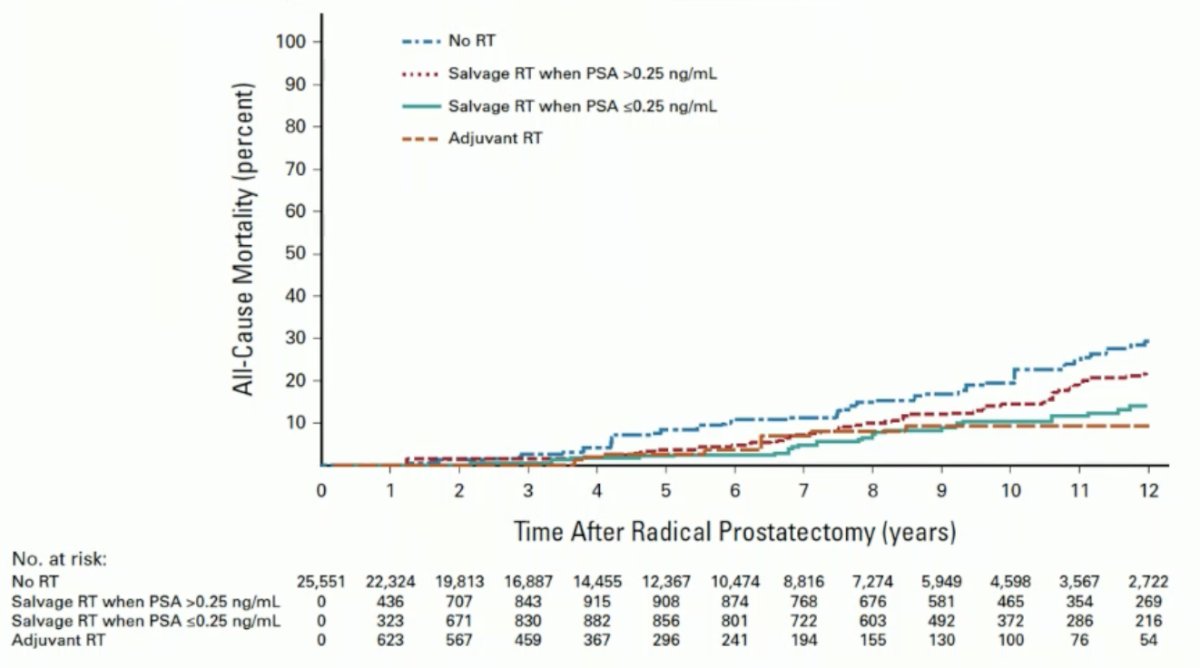
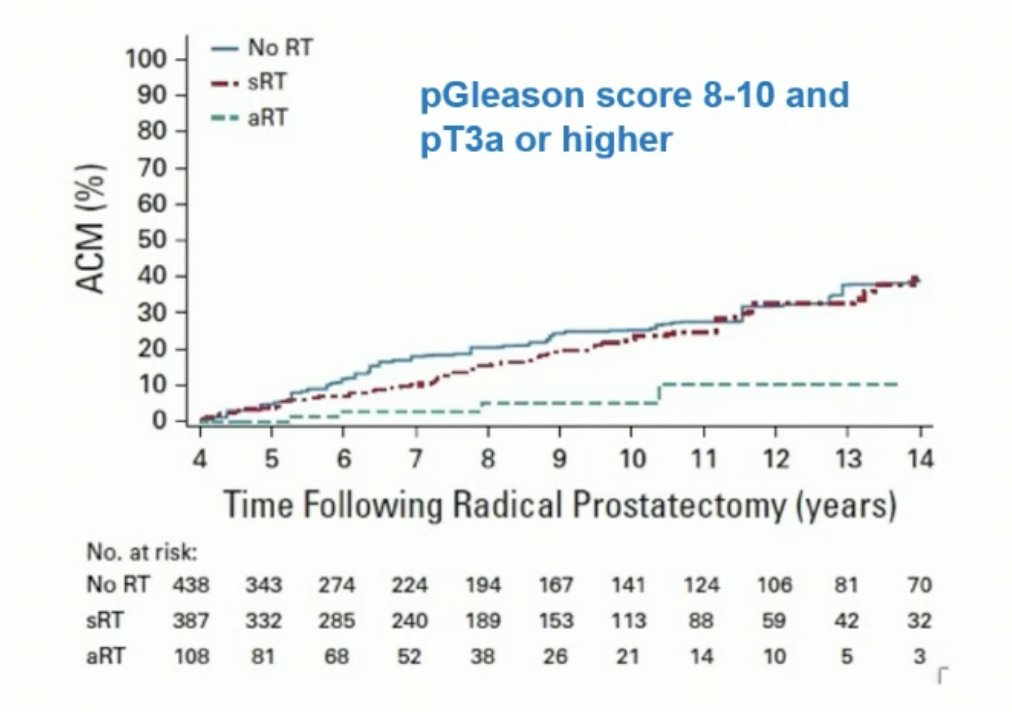
Data from three randomized controlled trials comparing adjuvant radiation therapy to early salvage radiotherapy (SRT) have emerged. A collaborative, prospectively designed systematic review and meta-analysis of these trials suggests that adjuvant radiotherapy does not improve event-free survival in men with localized or locally advanced prostate cancer. Until long-term outcome data are available, early salvage treatment appears to be the preferable approach.9 Dr. Tilki noted that these trials included only a minority of patients with adverse pathology, and the criteria for SRT differed between trials. A treatment propensity score cohort study comparing adjuvant radiotherapy with SRT in men with adverse pathology (pN1, pGleason score 8-10, pT3/4) found that adjuvant radiotherapy was associated with a significant reduction in all-cause mortality risk compared to early salvage radiation as shown in the graphic below.10 This is why the EAU still recommends adjuvant radiotherapy for selected patients with adverse pathology.
Dr. Tilki began by noting that salvage lymph node dissection (SLND) is not a standard treatment. The reported 5-year biochemical recurrence-free survival rates range from 6% to 31%, while the 5-year overall survival rate is approximately 84%, according to a systematic review of SLND. High-level evidence supporting the oncological value of salvage LN dissection is still lacking.11
A multicentric study including 189 patients with biochemical recurrence (BCR) and nodal-only recurrence after radical prostatectomy documented lymph node recurrence using PET/CT scans with either 11C-choline (81%) or 68Ga PSMA (3%). At a median follow-up of 87 months, 31% of patients were clinically recurrence-free, 11% were BCR-free, 18% were on ongoing androgen deprivation therapy (ADT), 24% had retroperitoneal disease, and the median PSA at the time of recurrence was 2.5 ng/ml. This study does not fully represent patients with "early" BCR.12
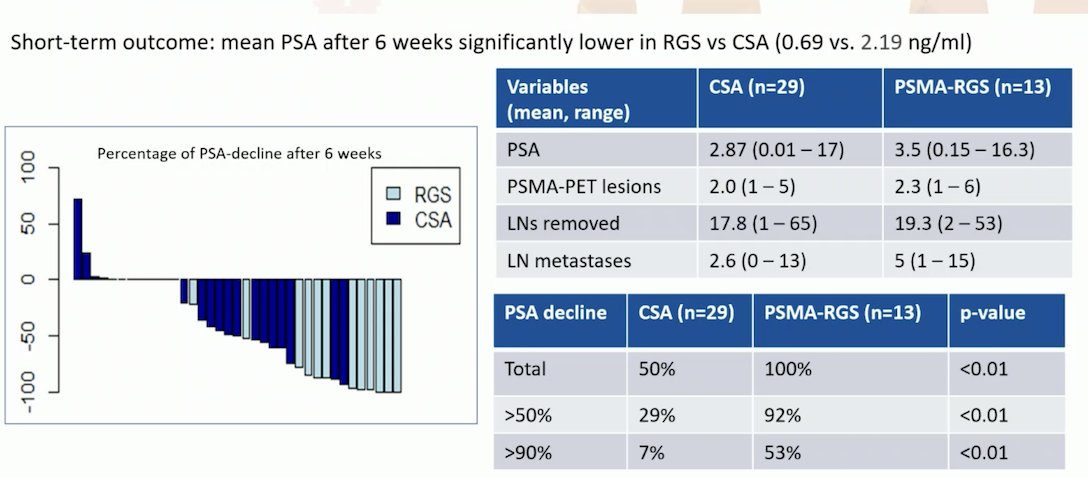
PSMA-radioguided surgery, which involves a preoperative intravenous injection of 99mTc-labeled PSMA ligands and intraoperative detection using a gamma probe with acoustic and visual feedback, has been compared to a conventional surgical approach guided by PSMA PET/CT imaging. The study compared 29 patients undergoing salvage lymph node dissection (SLND) based on PSMA PET/CT imaging with 13 patients receiving radioguided surgery. The short-term outcome showed that the mean PSA level after 6 weeks was significantly lower in patients who underwent radioguided surgery compared to those guided by PSMA PET/CT imaging (0.69 vs. 2.19 ng/ml). Additionally, a PSA decline of >90% was achieved in 53% of patients who received radioguided SLND, compared to 7% in those who underwent conventional SLND (p<0.01)13
The largest cohort of salvage lymph node dissection (SLND) was published by Knipper et al. The authors assessed 364 patients who underwent PET PSMA-guided SLND. Among these, 225 of 364 patients (approximately two-thirds) experienced biochemical recurrence (BCR), and 121 received further treatment. At 2 years, the biochemical recurrence-free survival rate was 32%. This underscores the argument that careful patient selection is crucial, based on life expectancy, PSA values, and the number of PSMA PET-avid lesions.
Dr. Tilki concluded her presentation with the following takeaway messages:
- Patients with biochemical recurrence after local treatment represent a heterogeneous population with diverse prognosis.
- The EAU prostate cancer guidelines recommend stratifying BCR patients into EAU low-risk and high-risk BCR groups.
- Most available studies included in systematic reviews did not use PET PSMA/Choline. PET can detect more metastases at BCR than conventional imaging and potentially improve oncologic outcomes.
- Limiting salvage treatments to patients who might benefit from them should be considered a priority to avoid overtreatment.
Presented by: Derya Tilki, MD, Martini-Klinik Prostate Cancer Center, University Hospital Hamburg-Eppendorf, Hamburg, Germany
Written by: Julian Chavarriaga, MD –Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:- Weiner AB, Kakani P, Armstrong AJ, Bossi A, Cornford P, Feng F, Kanabur P, Karnes RJ, Mckay RR, Morgan TM, Schaeffer EM, Shore N, Tree AC, Spratt DE. Risk Stratification of Patients with Recurrence After Primary Treatment for Prostate Cancer: A Systematic Review. Eur Urol. 2024 Sep;86(3):200-210. doi: 10.1016/j.eururo.2024.04.034. Epub 2024 May 22. PMID: 38782697.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.4.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed [September 14, 2024]. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Feng FY, Huang HC, Spratt DE, Zhao SG, Sandler HM, Simko JP, Davicioni E, Nguyen PL, Pollack A, Efstathiou JA, Dicker AP, Todorovic T, Margrave J, Liu YS, Dabbas B, Thompson DJS, Das R, Dignam JJ, Sweeney C, Attard G, Bahary JP, Lukka HR, Hall WA, Pisansky TM, Shah AB, Pugh SL, Shipley WU, Tran PT. Validation of a 22-Gene Genomic Classifier in Patients With Recurrent Prostate Cancer: An Ancillary Study of the NRG/RTOG 9601 Randomized Clinical Trial. JAMA Oncol. 2021 Apr 1;7(4):544-552. doi: 10.1001/jamaoncol.2020.7671. Erratum in: JAMA Oncol. 2021 Apr 1;7(4):639. doi: 10.1001/jamaoncol.2021.0552. PMID: 33570548; PMCID: PMC7879385.
- Ghadjar P, Hayoz S, Bernhard J, Zwahlen DR, Hölscher T, Gut P, Polat B, Hildebrandt G, Müller AC, Plasswilm L, Papachristofilou A, Schär C, Sumila M, Zaugg K, Guckenberger M, Ost P, Reuter C, Bosetti DG, Khanfir K, Gomez S, Wust P, Thalmann GN, Aebersold DM; Swiss Group for Clinical Cancer Research (SAKK). Dose-intensified Versus Conventional-dose Salvage Radiotherapy for Biochemically Recurrent Prostate Cancer After Prostatectomy: The SAKK 09/10 Randomized Phase 3 Trial. Eur Urol. 2021 Sep;80(3):306-315. doi: 10.1016/j.eururo.2021.05.033. Epub 2021 Jun 14. PMID: 34140144.
- Jani AB, Schreibmann E, Goyal S, Halkar R, Hershatter B, Rossi PJ, Shelton JW, Patel PR, Xu KM, Goodman M, Master VA, Joshi SS, Kucuk O, Carthon BC, Bilen MA, Abiodun-Ojo OA, Akintayo AA, Dhere VR, Schuster DM. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial. Lancet. 2021 May 22;397(10288):1895-1904. doi: 10.1016/S0140-6736(21)00581-X. Epub 2021 May 7. PMID: 33971152; PMCID: PMC8279109.
- Van den Broeck T, van den Bergh RC, Briers E, et al.. Biochemical recurrence in prostate cancer: the European Association of Urology prostate cancer guidelines panel recommendations. Eur Urol Focus. 2020;6:231–234
- Roberts MJ, Conduit C, Davis ID, Effeney RM, Williams S, Martin JM, Hofman MS, Hruby G, Eapen R, Gianacas C, Papa N, Lourenço RA, Dhillon HM, Allen R, Fontela A, Kaur B, Emmett L; Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP). The Dedicated Imaging Post-Prostatectomy for Enhanced Radiotherapy outcomes (DIPPER) trial protocol: a multicentre, randomised trial of salvage radiotherapy versus surveillance for low-risk biochemical recurrence after radical prostatectomy. BJU Int. 2024 Feb;133 Suppl 3:39-47. doi: 10.1111/bju.16158. Epub 2023 Sep 14. PMID: 37604702.
- Tilki D, Chen MH, Wu J, Huland H, Graefen M, Mohamad O, Cowan JE, Feng FY, Carroll PR, D'Amico AV. Prostate-Specific Antigen Level at the Time of Salvage Therapy After Radical Prostatectomy for Prostate Cancer and the Risk of Death. J Clin Oncol. 2023 May 1;41(13):2428-2435. doi: 10.1200/JCO.22.02489. Epub 2023 Mar 1. PMID: 36857638; PMCID: PMC10150889.
- Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, Sargos P, Sydes MR, Brawley C, Brihoum M, Brown C, Chabaud S, Cook A, Forcat S, Fraser-Browne C, Latorzeff I, Parmar MKB, Tierney JF; ARTISTIC Meta-analysis Group. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020 Oct 31;396(10260):1422-1431. doi: 10.1016/S0140-6736(20)31952-8. Epub 2020 Sep 28. PMID: 33002431; PMCID: PMC7611137.
- Tilki D, Chen MH, Wu J, Huland H, Graefen M, Wiegel T, Böhmer D, Mohamad O, Cowan JE, Feng FY, Carroll PR, Trock BJ, Partin AW, D'Amico AV. Adjuvant Versus Early Salvage Radiation Therapy for Men at High Risk for Recurrence Following Radical Prostatectomy for Prostate Cancer and the Risk of Death. J Clin Oncol. 2021 Jul 10;39(20):2284-2293. doi: 10.1200/JCO.20.03714. Epub 2021 Jun 4. PMID: 34086480.
- Ploussard, G., et al. Salvage Lymph Node Dissection for Nodal Recurrent Prostate Cancer: A Systematic Review. Eur Urol, 2019. 76: 493.
- Bravi CA, Fossati N, Gandaglia G, Suardi N, Mazzone E, Robesti D, Osmonov D, Juenemann KP, Boeri L, Jeffrey Karnes R, Kretschmer A, Buchner A, Stief C, Hiester A, Nini A, Albers P, Devos G, Joniau S, Van Poppel H, Shariat SF, Heidenreich A, Pfister D, Tilki D, Graefen M, Gill IS, Mottrie A, Karakiewicz PI, Montorsi F, Briganti A. Long-term Outcomes of Salvage Lymph Node Dissection for Nodal Recurrence of Prostate Cancer After Radical Prostatectomy: Not as Good as Previously Thought. Eur Urol. 2020 Nov;78(5):661-669. doi: 10.1016/j.eururo.2020.06.043. Epub 2020 Jul 2. PMID: 32624288; PMCID: PMC9084633.
- Knipper S, Ascalone L, Ziegler B, Hohenhorst JL, Simon R, Berliner C, van Leeuwen FWB, van der Poel H, Giesel F, Graefen M, Eiber M, Heck MM, Horn T, Maurer T. Salvage Surgery in Patients with Local Recurrence After Radical Prostatectomy. Eur Urol. 2021 Apr;79(4):537-544. doi: 10.1016/j.eururo.2020.11.012. Epub 2020 Dec 11. PMID: 33317857.


