(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a session on future therapy targets for metastatic prostate cancer patients. Dr. Himisha Beltran discussed the potential of Delta-like ligand 3 (DLL3) as a therapeutic target for neuroendocrine prostate cancer.
Why target cell surface proteins? This allows for the development of new and improved compounds that facilitate drug delivery, potentially via antibody-drug conjugates and radionuclide therapies, as well as T-cell engagement, possible via re-directing/activating T cells and the immune response. This also has potential biomarker implications, whereby we can measure the target. Examples of this include PET scans and tissue immunohistochemistry. This may facilitate a precision medicine approach, whereby we only treat those cells that express the target. Targeting cell surface proteins may also be a mechanism to target intrinsic drivers and activate downstream pathways (e.g., HER2/3 antibody drug conjugates in breast cancer).
A well-known cell surface protein target in prostate cancer is prostate-specific membrane antigen (PSMA), which is a well-established imaging and theranostic target in this space. Other emerging cell surface proteins in prostate cancer include DLL-3, STEAP1, b7-h3, PSCA, TROP2, KLK2, CEACAM5, and HER2/3.
These different targets have similar considerations, namely:
- Target expression, regulation, and dynamics: treat all or select?
- Drug properties: How much target is required?
- Mechanism: Payload is important for response/resistance
- Resistance: Informs combination strategies and drug sequencing
Delta-like protein 3 is not expressed in benign tissues and is rarely expressed in localized prostate cancer. Its expression increases in castrate-resistant prostate cancer (12.5%). However, when prostate cancer cells undergo neuroendocrine diversion in the castrate-resistant setting, its expression increases to 76.6%.1
Dr. Betran noted that neuroendocrine prostate cancer rarely arises ‘de novo’, but often is a product of lineage plasticity reflecting an adaptive mechanism of treatment resistance in later stages of prostate cancer. These cells are classically resistant to androgen inhibition and have low PSMA expression.
Delta-like protein 3 (DLL3) encodes a member of the delta protein-ligand family involved in Notch signaling (ligand inhibitor). DLL3 is expressed in the developing brain, thymus, and paraxial mesoderm and plays a key role in somitogenesis.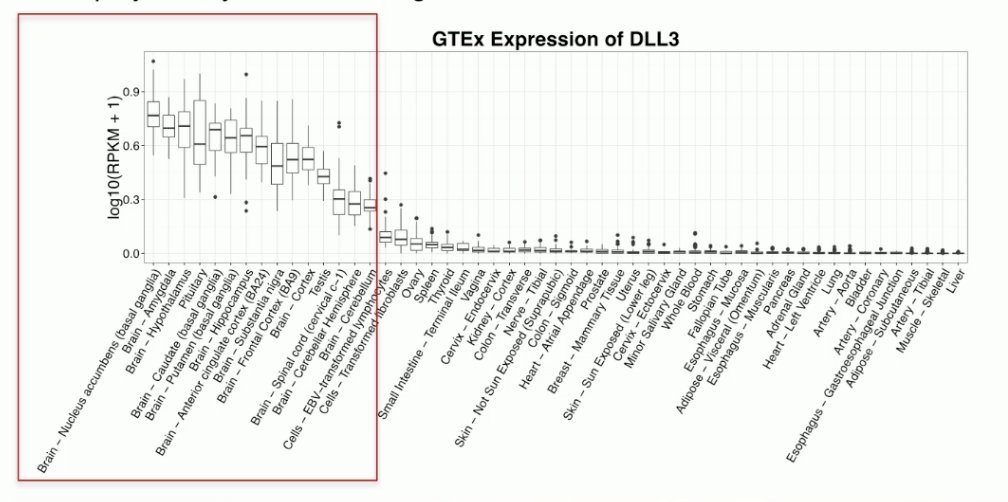
During normal neuronal development Notch signaling is ‘on”. When stem cells have matured, Notch signaling is turned ‘off’. Prostate cancer neuroendocrine tumors share similar known features as neuronal cells.
Dr. Beltran’s group recently discovered that Notch signaling can be oncogenic in early prostate cancer but is also ‘shut down’ as tumors transform from adenocarcinoma to neuroendocrine prostate cancer, becoming tumor suppressive. This corresponds with overexpression of DLL3 and ASCL1, which are important for maintaining a neuroendocrine phenotype.2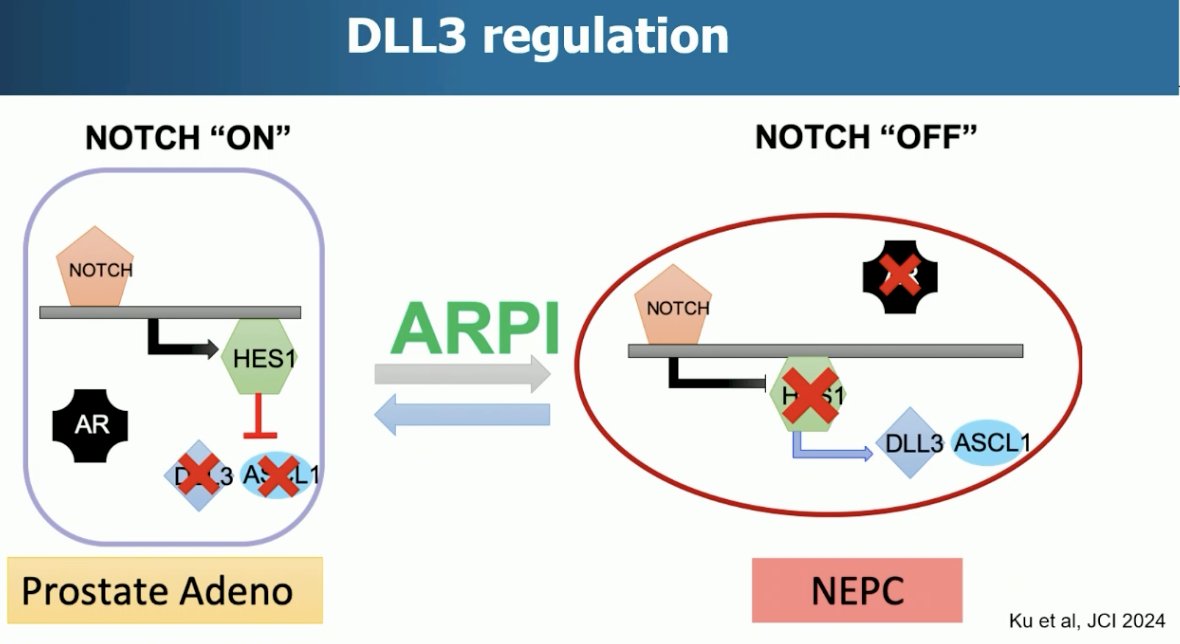
DLL3 is also expressed in other neuroendocrine carcinomas, as illustrated below:
An early DLL3 targeted antibody-drug conjugate, Rova-T, demonstrated promising pre-clinical activity and early-phase clinical activity in small-cell lung cancer. However, Rova-T failed in two phase III trials (MERU and TAHOE), with efficacy limited and high toxicity. The development of Rova-T was discontinued in 2018.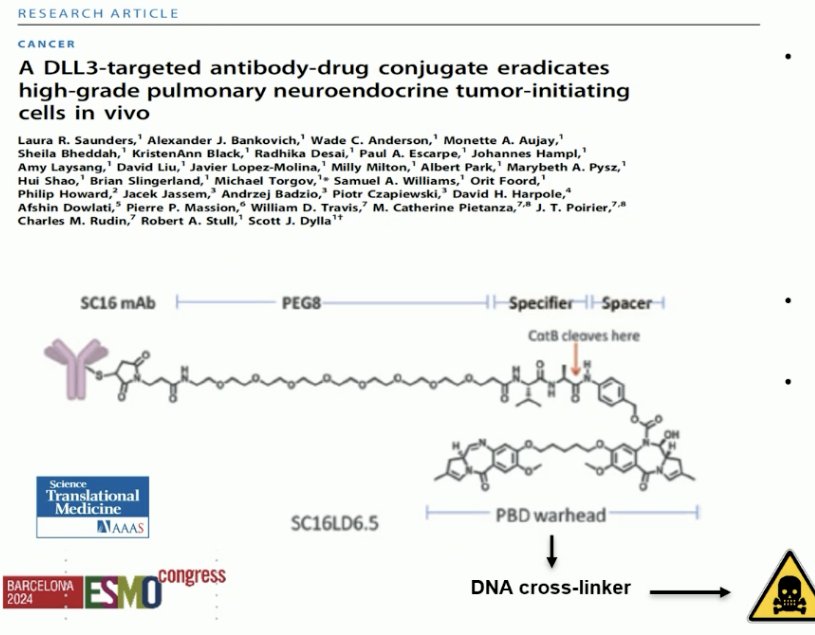
However, there are now numerous ongoing DLL3-targeted therapies in development, including:
- DLL3 targeted T cell engagers
- DLL3 next-generation ADCs
- DLL3 radionuclides
- DLL3 bi-specifics with other partners (e.g., CD47)
- DLL3 CAR-T
- Others
Tarlatamab is a DLL3 targeted, bi-specific T cell engager approved in May 2024 for previously treated small cell lung cancer patients.
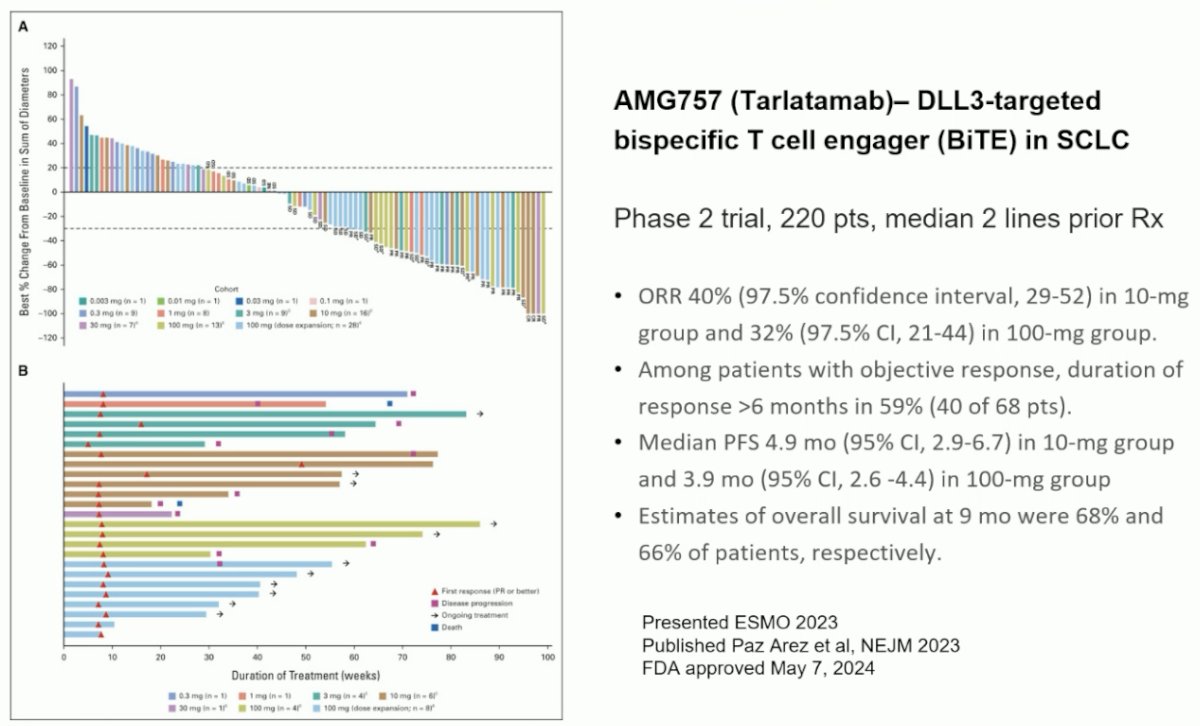
Should we be using tarlatamab for neuroendocrine prostate cancer? The current NCCN guidelines recommend platinum-based chemotherapy combinations for the treatment of small cell/neuroendocrine prostate cancer.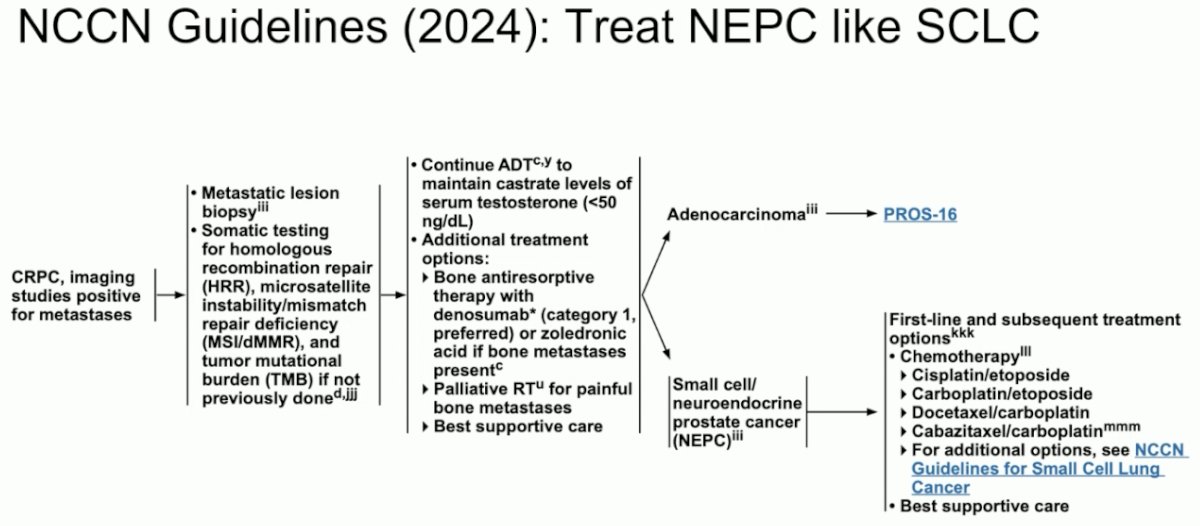
The results of a study evaluating tarlatamab in neuroendocrine prostate cancer were presented at ASCO 2024. This trial included patients with de novo or treatment-emergent neuroendocrine prostate cancer with ≥2 alterations in TP53, RB1, and/or PTEN by immunohistochemistry or genomic analysis and progressed on ≥1 prior systemic treatment (platinum chemotherapy or androgen signaling inhibitor). In this trial, patients received tarlatamab 100 mg IV every two weeks with one-step dosing, as summarized below: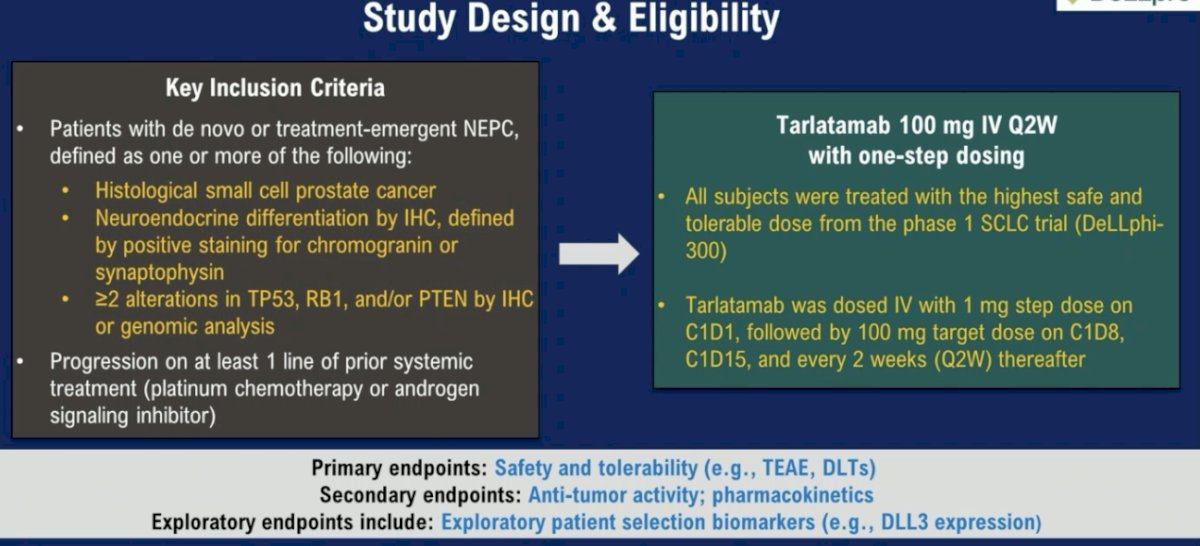
Efficacy outcomes were disappointing. The objective response rate in the overall cohort was 10.5%, and 22.2% in the DLL3+ cohort. The median duration of treatment was only 1.4 months overall (3.6 months in the DLL3+ cohort). The median duration of response was 7.3 months; 1 patient remains on treatment with an ongoing response at 25.8+ months. Dr. Beltran noted that only 56% of patients were DLL3+, and DLL3+ positivity was defined as ≥1% DLL3 tumor positivity by immunohistochemistry. As such, she argued that this may not be the ‘prime’ cohort of patients to benefit from such treatment.
MK-6070 (formerly HPN328) is a DLL3-targeting T cell engager with strong activity in DLL3+ cancer models and has demonstrated anti-tumor activity in vivo. When DLL3+ patient-derived neuroendocrine prostate cancer xenografts were injected into mice models, HPN328 co-injected with human T cells resulted in tumor regression and prolonged survival of mice.
The initial results of an ongoing phase I/II trial of MK-6070 was presented by Dr. Beltran at ADCO GU 2024. This is a phase I/II study that employed a 3+3 dose escalation design for a total of 85 patients, of whom patients with neuroendocrine prostate cancer accounted for 15/85 (other tumors included small cell lung and other neuroendocrine cancers). MK-6070 was administered weekly or bi-weekly. The target population was:
- Neuroendocrine prostate cancer relapsed or refractory to standard of care
- Other DLL3+ high-grade neuroendocrine neoplasms
- Small cell lung cancer relapsed/refractory to platinum chemotherapy
The trial objectives were to:
- Assess safety and tolerability
- Determine the recommended phase 2 dose (RP2D) and/or the maximum tolerated dose (MTD)
- Characterize pharmacokinetics and pharmacodynamics
- Evaluate preliminary anti-tumor activity
Anti-tumor activity in 1 mg priming subjects evaluable for response is summarized below. Among the 12 evaluable GU neuroendocrine cancer patients, the overall response rate was 58%, with a disease control rate of 83%. 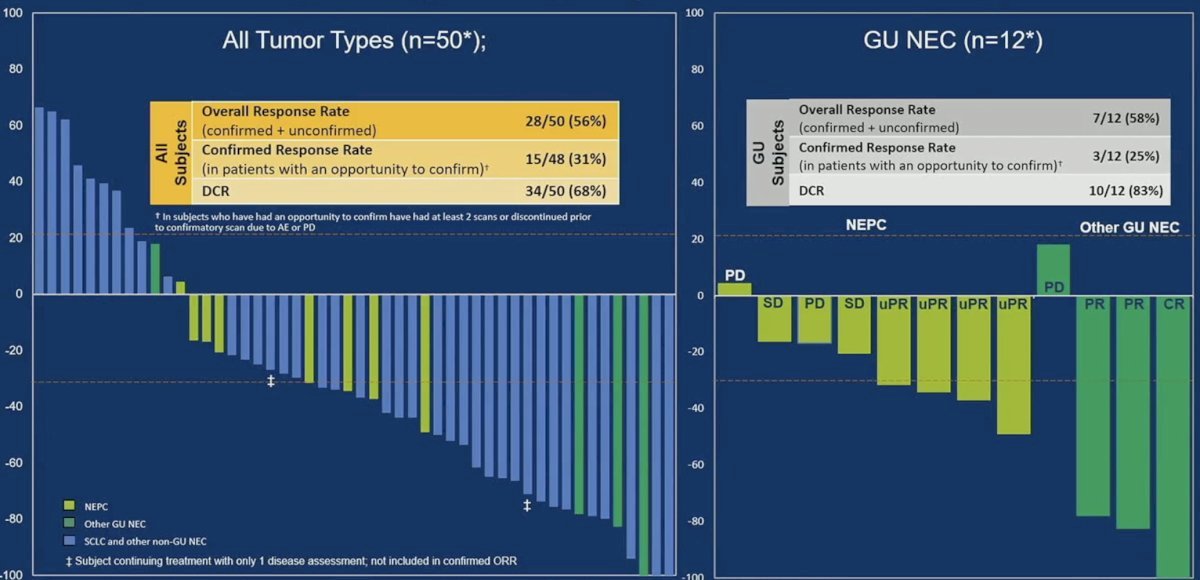
The swimmer’s plot below illustrates the duration of response and treatment among 17/21 GU neuroendocrine patients. One patient receiving 6 mg once weekly has an ongoing response at >50 weeks of treatment. 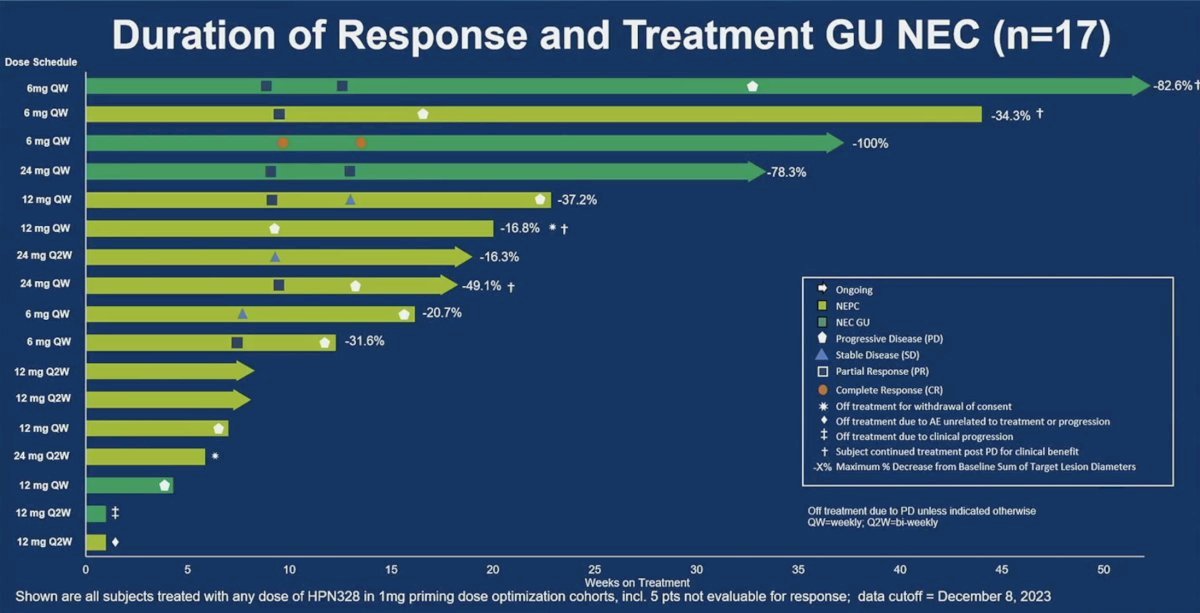
Can we use DLL3 for PET imaging? 89Zr-SC16 is the first PET scan tracer for detecting and quantifying in vivo tumor DLL3 expression. In the future, such PET imaging may be used to complement PSMA PET imaging for detecting adenocarcinoma and neuroendocrine patterns, selecting patients for DLL-3 targeted therapy, and understanding resistance patterns.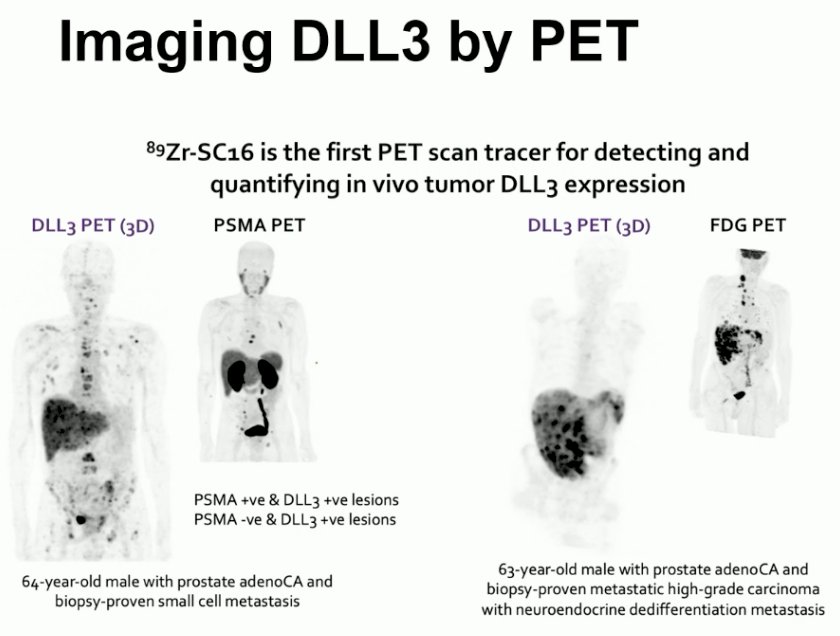
Dr. Beltran concluded as follows:
- DLL3 has emerged as a therapeutic target for small cell/neuroendocrine carcinomas – a very aggressive subset of cancers, including neuroendocrine prostate cancer
- DLL3-targeted T cell engagers have demonstrated activity in small cell/neuroendocrine carcinomas. Other DLL3-targeted drugs are also in development.
- For prostate cancer, patient selection is important. DLL3 is not expressed in most prostate adenocarcinomas, and therefore heterogeneous tumor features can lead to mixed responses.
- Biomarkers that capture heterogeneity (e.g., imaging, cfDNA) may help improve patient selection in the future and inform rational combination strategies.
- Understanding resistance patterns could inform next-line therapy (DLL3, other)
Presented by: Himisha Beltran, MD, Associate Professor of Medicine, Department of Medical Oncology in the Lank Division of Genitourinary Oncology and the Division of Molecular and Cellular Oncology at Dana Farber Cancer Institute and Harvard Medical School, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: Emerging DLL3-Targeted Therapies Demonstrate Efficacy in Neuroendocrine Prostate Cancer - Himisha Beltran
References:
- Puca L, Gavyert K, Sailer V, et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci Transl Med. 2019; 11(484):eaav0891.
- Ku SY, Wang Y, Garcia MM, et al. Notch signaling suppresses neuroendocrine differentiation and alters the immune microenvironment in advanced prostate cancer. J Clin Invest. 2024; 134(17):e175217.


