(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a proffered paper session for prostate cancer. Dr. Arun Azad presented the results of UpFrontPSMA, a randomized phase II trial of sequential 177Lu-PSMA-617 plus docetaxel versus docetaxel in metastatic hormone-sensitive prostate cancer (mHSPC). This study was concurrently published in The Lancet Oncology on September 15, 2024.

177Lu-PSMA-617 is approved and recommended in mCRPC,1,2 but its utility in hormone-sensitive disease remains uncertain. The CHAARTED trial established androgen deprivation therapy (ADT) + docetaxel as a standard of care for de novo high-volume mHSPC.3,4 Nevertheless, outcomes remain poor for this patient cohort.
The study investigators hypothesized that 177Lu-PSMA-617 followed by docetaxel would enhance outcomes with minimal impact on toxicity in de novo, PSMA PET-defined high-volume mHSPC.
The study design is summarized below. This trial included patients with de novo high-volume mHSPC who had received ≤4 weeks of ADT and had a PSA >10 ng/ml at diagnosis. Prior to randomization patients underwent both PSMA and FDG PET scans. Eligibility was limited to those patients with evidence of high tumor uptake and high-volume disease on PET scans. Patients were also required to have the majority of their metastatic disease demonstrating PSMA positivity. Eligible patients were randomized to:
- Experimental arm: 177Lu-PSMA-617 7.5 GBq x 2 cycles + docetaxel 75 mg/m2 x 6 cycles
- Control arm: Docetaxel 75 mg/m2 x 6 cycles
The primary endpoint was undetectable PSA at 48 weeks, defined as a PSA ≤0.2 ng/ml. Secondary endpoints included:
- PSA progression-free survival
- Development of castration resistance
- Radiographic progression-free survival
- Overall survival
- Quality of life and pain
- Adverse events

A sample size of 140 patients was initially chosen to provide 85% power assuming an undetectable PSA proportion at 48 weeks of 25% in the control arm and 50% in the experimental arm. For reference, in CHAARTED, 27% of patients in the ADT + docetaxel arm had an undetectable PSA at 12 months. Accrual was stopped at 130 patients due to delays in recruitment caused by COVID-19-related lockdowns in Australia in 2020 and 2021. The power was reduced from 85% with 140 patients to 82% with 130 patients.
Between May 2020 and April 2023, 130 patients were recruited and underwent randomization (experimental: 63, control: 67). Overall, 22% of screened patients failed on PET. The median follow-up was 2.5 years.
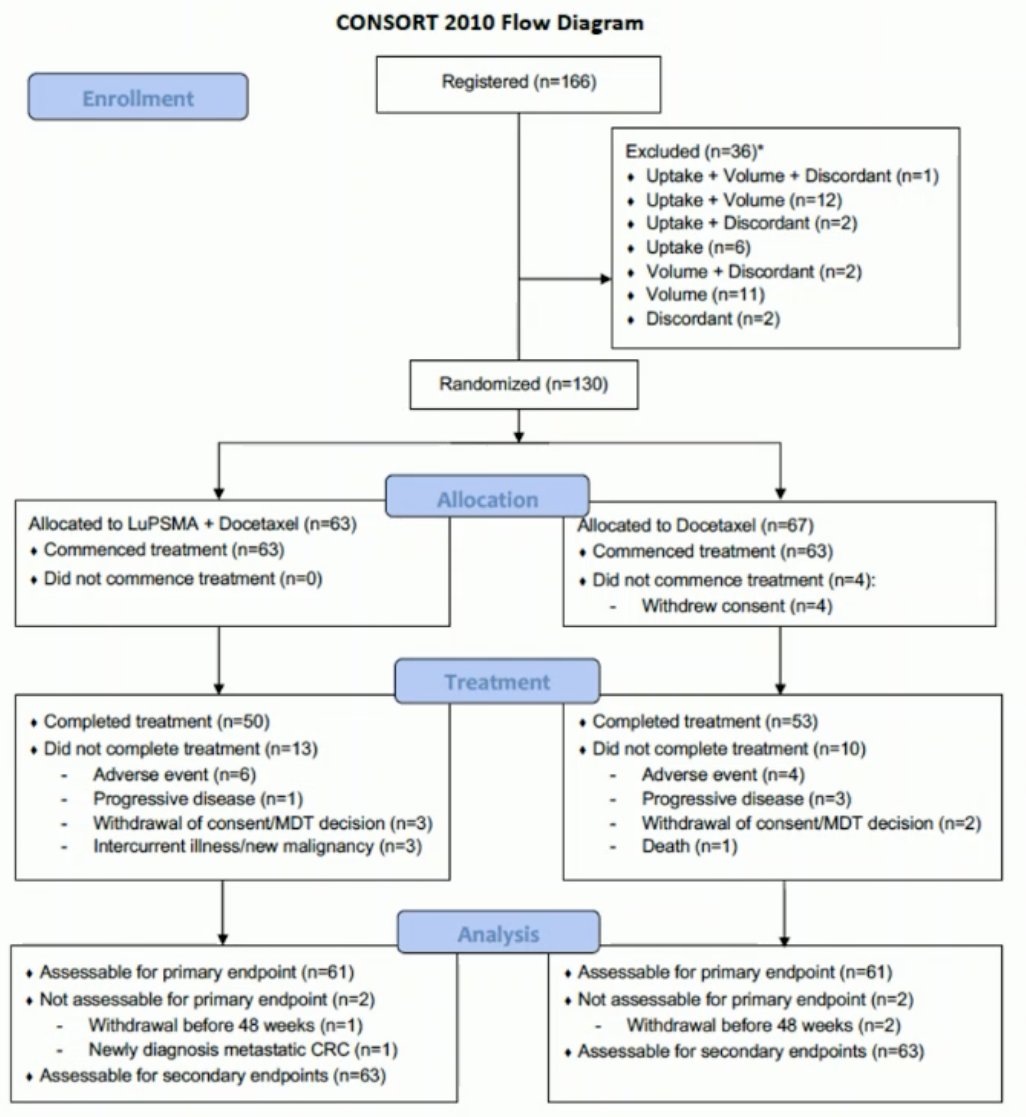
All 63 patients in the experimental arm completed the two cycles of 177Lu-PSMA. 79% of patients in the combination arm completed all 6 cycles of docetaxel, compared to 84% of patients in the control arm. However, a docetaxel dose reduction was required in 33% of patients in the combination arm, compared to 17% of patients in the docetaxel arm.

The median patient age of the cohort was 69 years. 8% of patients had visceral disease. Grade Group 4–5 disease was present in 91% of cases at diagnosis. The median PSA was higher in the 177Lu-PSMA + docetaxel arm (48 versus 31 ng/ml). 91% of patients had high-volume disease on conventional imaging (95% versus 87%, respectively).

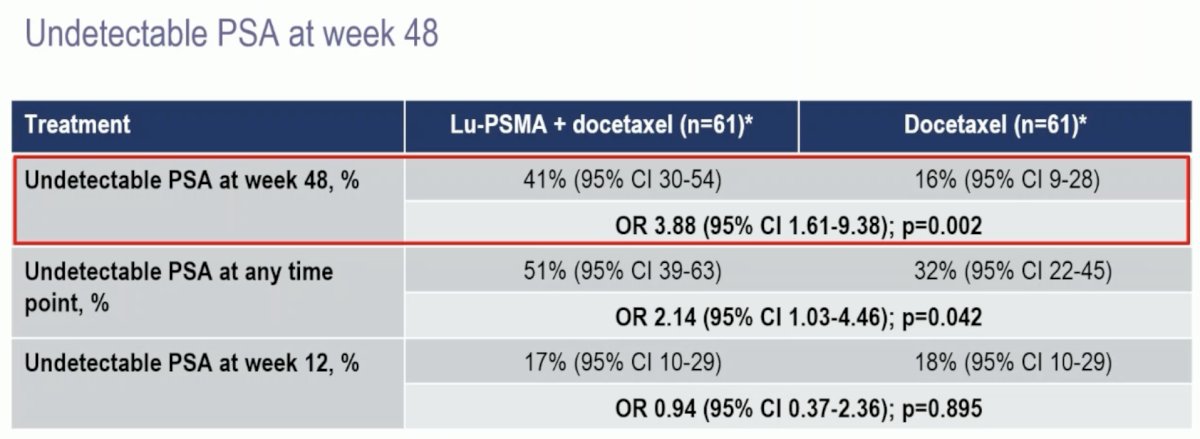
With regards to the primary outcome, an undetectable PSA at week 48 was observed in 41% of patients in the 177Lu-PSMA + docetaxel arm versus 16% of patients in the docetaxel control arm (OR: 3.88, 95% CI: 1.61–9.38, p=0.002). An undetectable PSA at any point was observed in 51% and 32% of patients, respectively (OR: 2.14, p=0.042). There were no differences in undetectable PSA levels at week 12 between the two arms (p=0.9).
Time-to-event analyses demonstrated that patients in the experimental arm had superior PSA progression-free survival (median: 31 versus 20 months; HR: 0.60, p=0.039) and freedom from castration resistance (HR: 0.60, p=0.033).

Radiographic progression-free survival similarly favored 177Lu-PSMA + docetaxel (HR: 0.58, p=0.067). To date, there is no overall survival benefit with the addition of 177Lu-PSMA to docetaxel (HR: 0.83, 95% CI: 0.38–1.83, p=0.646).

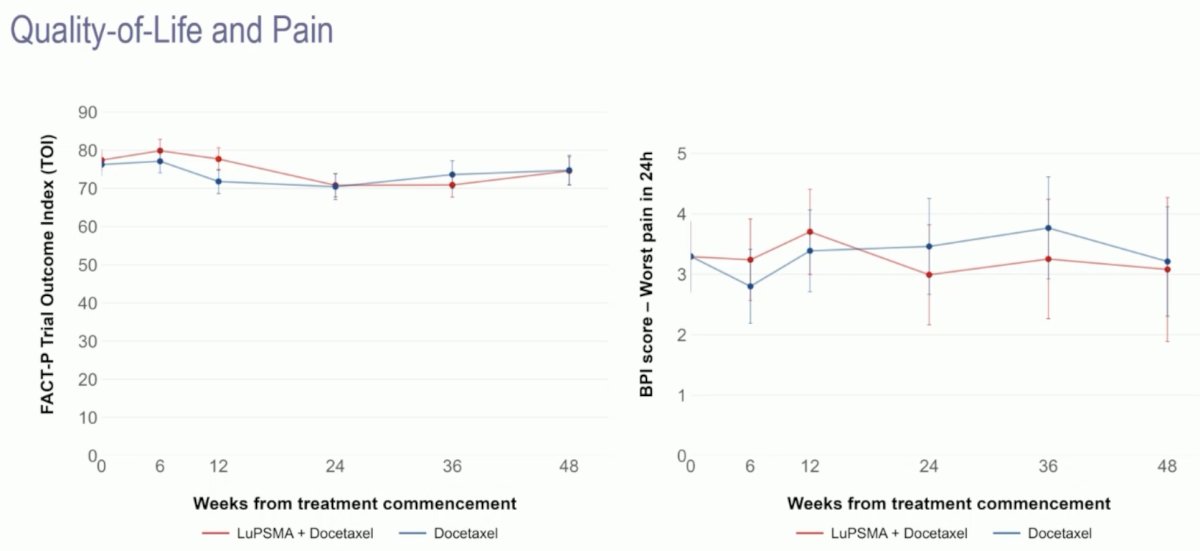
Quality of life and pain scores were stable throughout the study period.
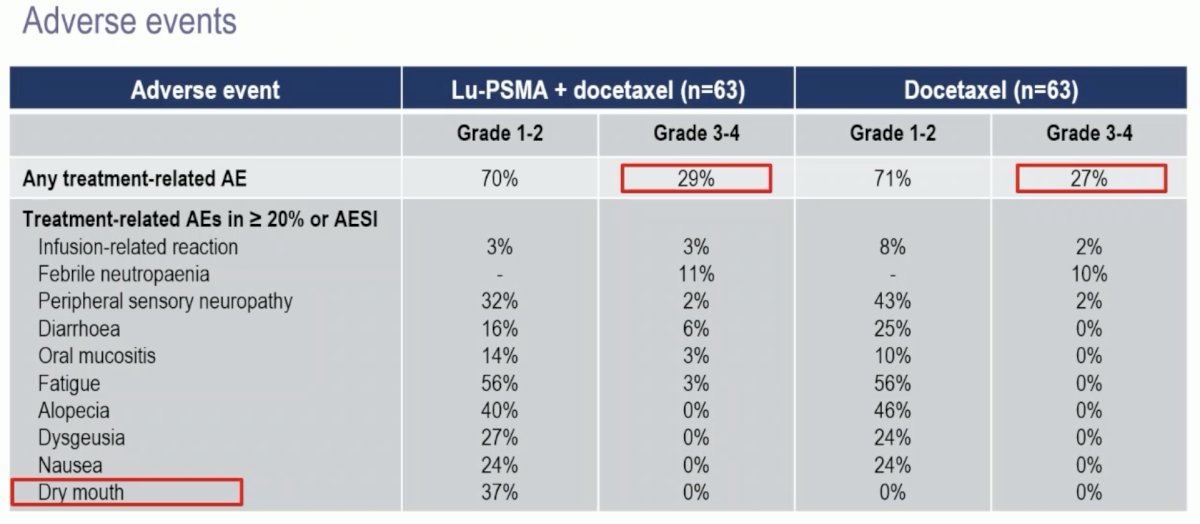
Adverse events were similar in both arms, with grade 3–4 events in 27–29% of cases. There were no new safety signals. As expected, patients in the 177Lu-PSMA arm had a higher frequency of dry mouth (37% versus 0% – all grade 1–2).
Dr. Azad concluded as follows:
- UpFrontPSMA is the first randomized study in mHSPC showing a benefit from the addition of 177Lu-PSMA-617 to standard-of-care treatment
- In patients with de novo high-volume mHSPC, 177Lu-PSMA-617 followed by docetaxel, as compared to docetaxel alone, significantly improved the primary endpoint of undetectable PSA at 48 weeks with no increase in overall toxicity
- These data indicate that 177Lu-PSMA-617 potentially has a role in the therapeutic management of mHSPC
- The phase 3 PSMAddition study will further inform on the utility of 177 Lu-PSMA-617 in mHSPC
Presented by: Arun Azad, PhD, MBBS, FRACP, Associate Professor, Department of Medicine, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021; 397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021; 385(12):1091-103.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016; 387(10024):1163-77.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results in the STAMPEDE trial. Ann Oncol. 2019; 30(12):1992-2003.


