(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a proffered paper session for prostate cancer. Dr. Oliver Sartor presented the first interim results of SPLASH, a phase III trial of 177Lu-PNT2002 in PSMA-positive metastatic castrate-resistant prostate cancer (mCRPC) following progression on an androgen-receptor pathway inhibitor (ARPI).
177Lu-PNT2002 ([Lu 177]-PSMA-I&T) is a prostate specific membrane antigen (PSMA)-targeted small molecule radioligand linked to a DOTAGA radiometal chelator. SPLASH (NCT04647526) is a phase Ill, randomized study designed to evaluate the efficacy and safety of 177Lu-PNT2002 in mCRPC patients who experience disease progression following an ARPI.
177Lu-PNT2002 delivers PSMA-targeted radiation to prostate cancer cells, causing DNA damage and ultimately cancer cell death.

The SPLASH study design is summarized below. This trial included patients with progressive mCRPC with disease progression on prior treatment with one ARPI. All patients were required to have PSMA-avid PET disease. Taxane chemotherapy for castrate-sensitive disease was allowed, as long as it was >1 year prior to consent. Eligible patients were randomized 2:1 to:
- Experimental arm: 177Lu-PNT2002 6.8 GBq (+/- 10%) every 8 weeks for 4 cycles
- Control arm: Alternate ARPI (enzalutamide or abiraterone)
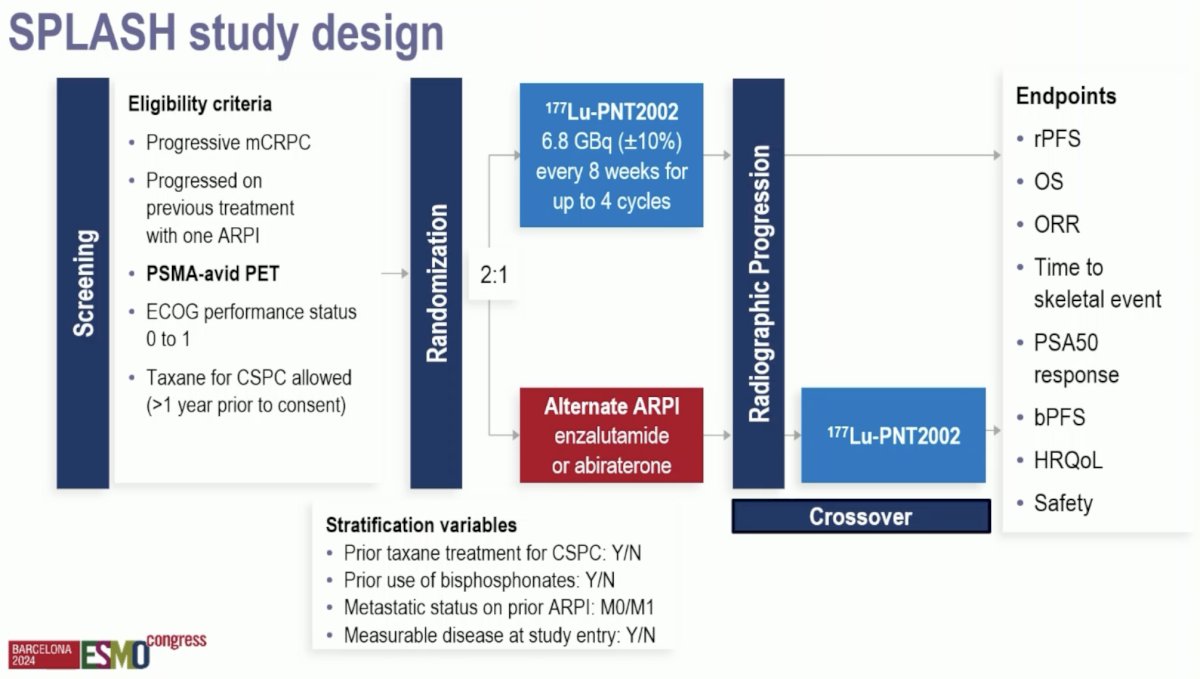
The primary endpoint was radiographic progression-free survival. Notably, at radiographic progression in the control arm, crossover to 177Lu-PNT2002 was permitted. Secondary endpoints included:
- Overall survival
- Objective response rate
- Time to skeletal event
- PSA50 response
- Health related quality of life
- Safety
A total of 412 patients were deemed eligible and randomized to either the experimental (n=276) or control arm (n=136). Notably, 85% of patients in the control arm of alternate ARPI crossed over to the experimental treatment of 177Lu-PNT2002 at radiographic progression.

The baseline patient characteristics are summarized below. The median patient age was 72 years. The median PSA was lower in the experimental arm (13.2 versus 19 ng/ml). Approximately 17% of patients had received prior taxanes for castrate-sensitive disease. Most patients (90%) had received the prior ARPI therapy in an M1 state.

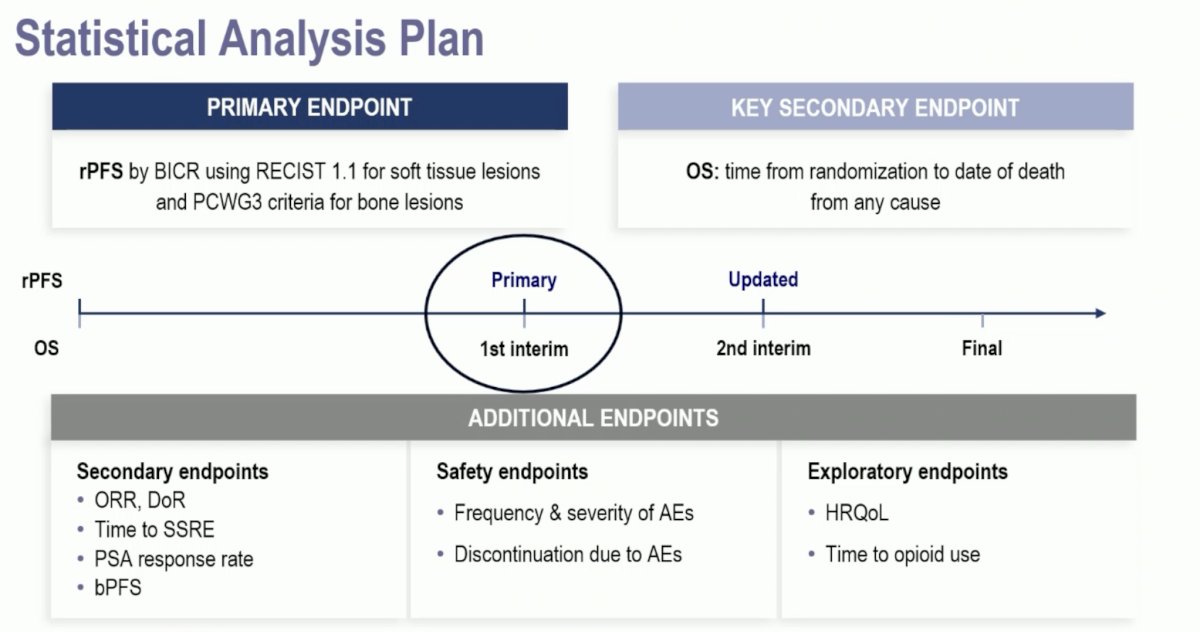
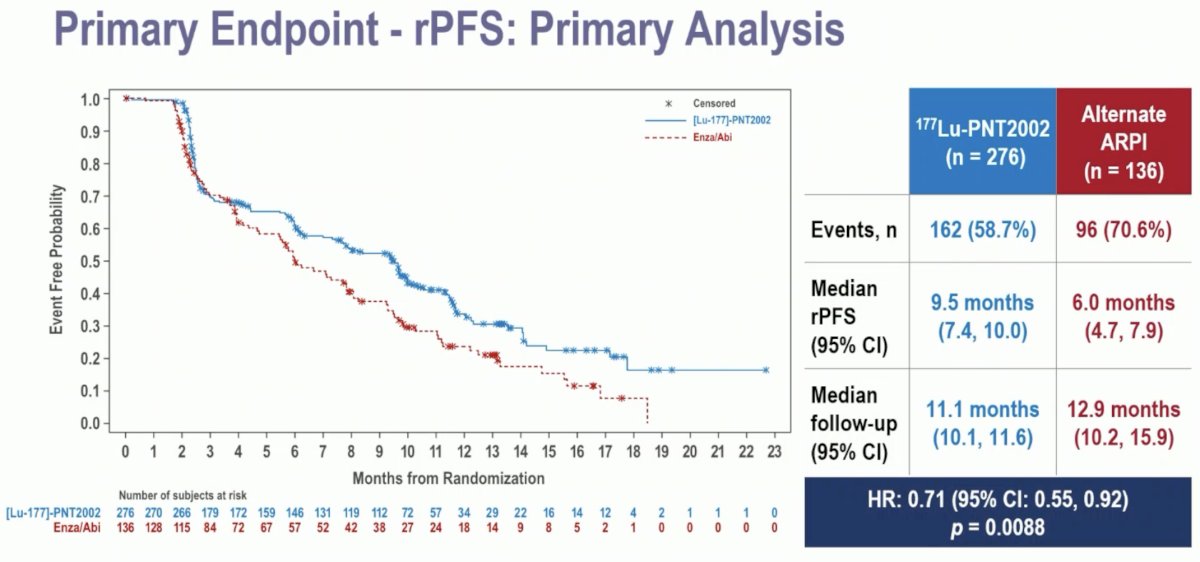
At the data cut-off date of November 1st, 2023, with a median follow-up of ~12 months, this trial met its primary endpoint of a radiographic progression-free survival benefit with 177Lu-PNT2002 (median: 9.5 versus 6 months; HR: 0.71, 95% CI: 0.55–0.92, p=0.0088).
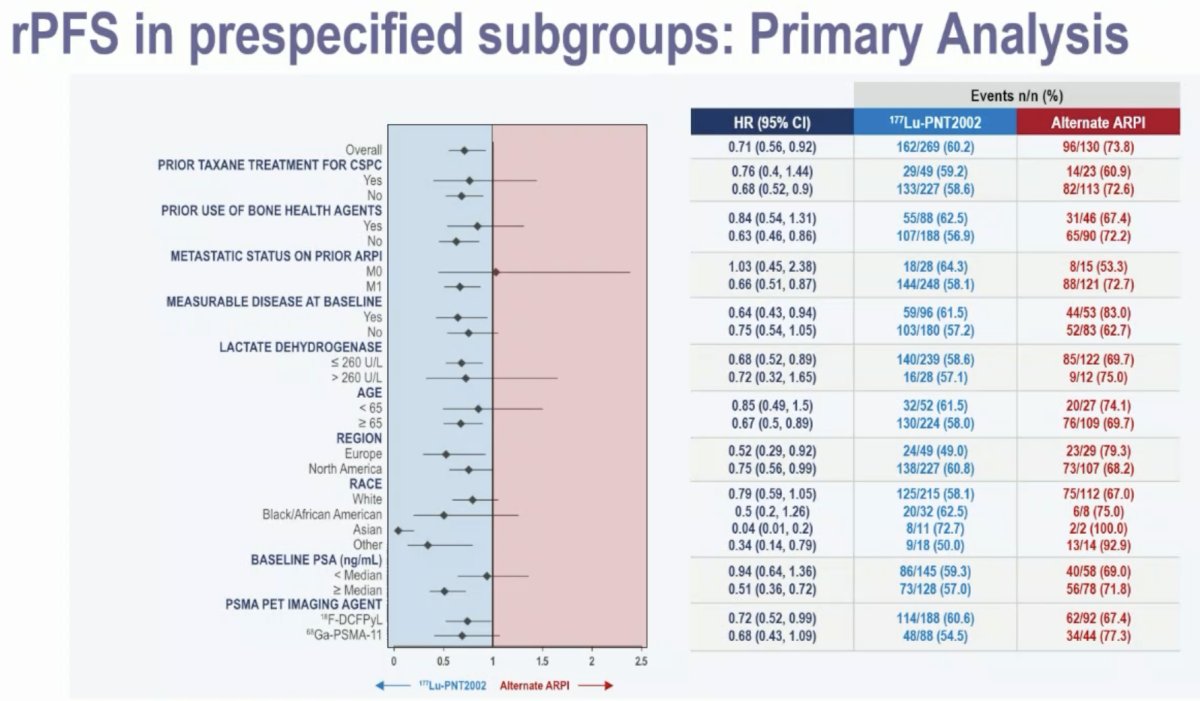
Subgroup analyses demonstrated consistent radiographic progression-free survival benefits with 177Lu-PNT2002 across the evaluated strata.
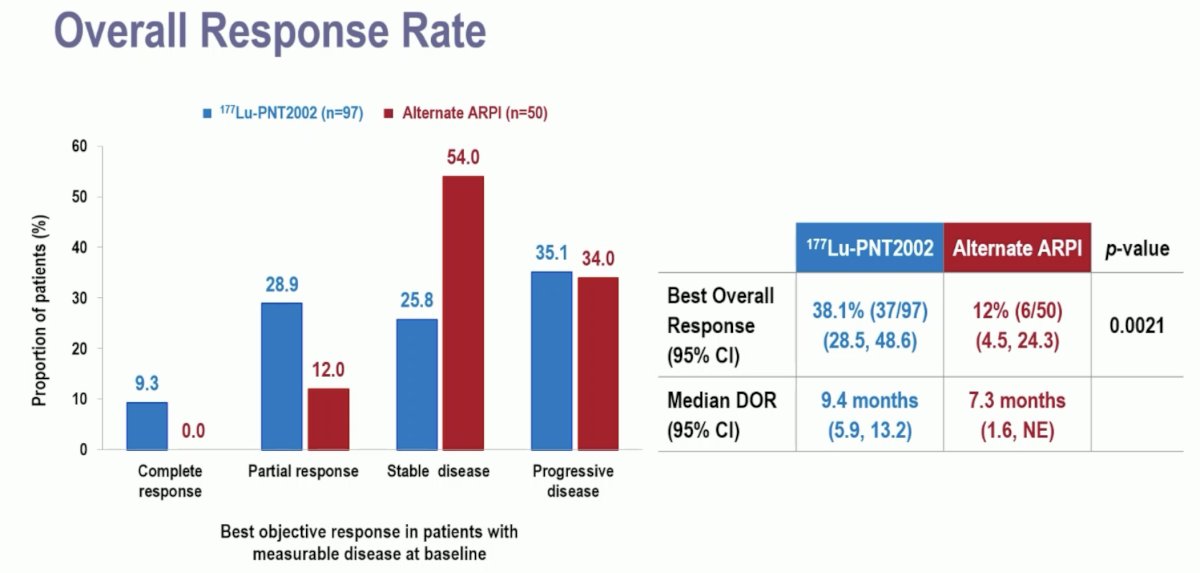
A complete response was observed in 9.3% of patients in the experimental arm, compared to none in the control arm. The best overall response was observed in 38% and 12% of patients, respectively (p=0.0021). The median duration of responses was 9.4 and 7.3 months, respectively.
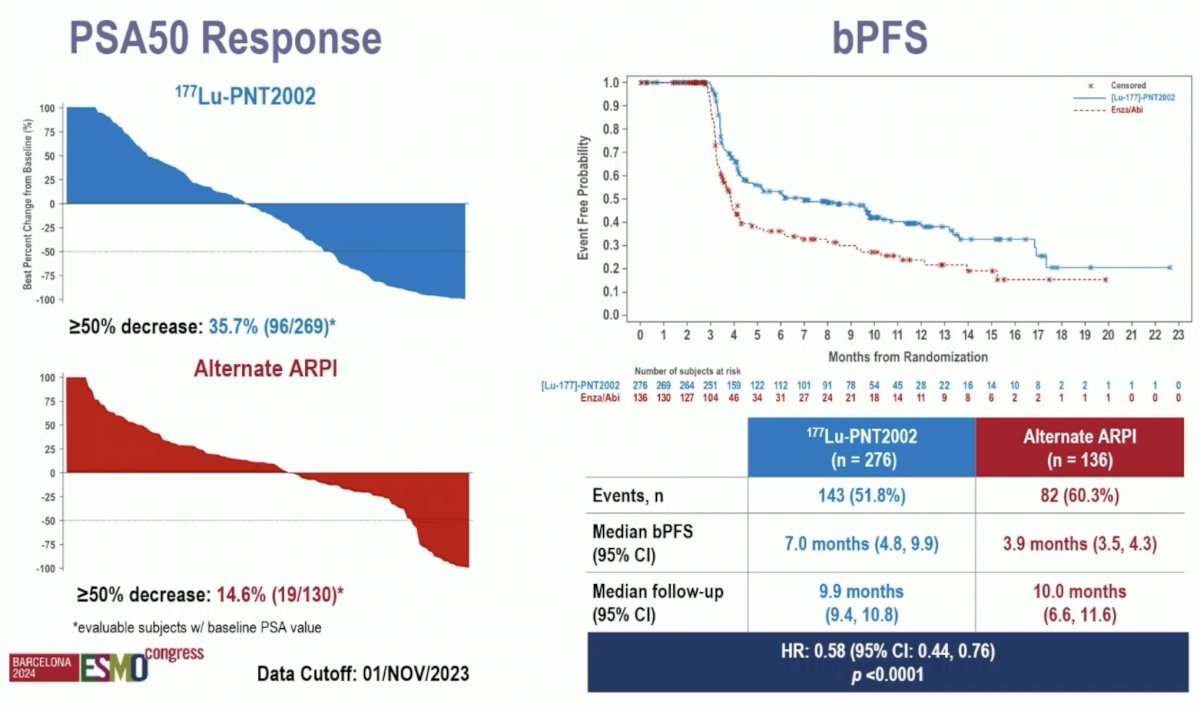
A PSA50 response was observed in 36% and 15% of patients, respectively. Biochemical progression-free survival similarly favored 177Lu-PNT2002 (median: 7 versus 3.9 months; HR: 0.58, 95% CI: 0.44–0.76, p<0.001).
Health related quality of life deterioration, assessed via the FACT-P questionnaire, favored the 177Lu-PNT2002 arm (HR: 0.59, p=0.0005). Time to opioids consumption similarly favored the 177Lu-PNT2002 arm (HR: 0.64, p=0.037).

The 1st interim analysis of overall survival did not demonstrate an overall survival benefit for 177Lu-PNT2002 (median: 20.8 months versus non-estimable; HR: 1.11, 95% CI: 0.73–1.69, p=0.62).
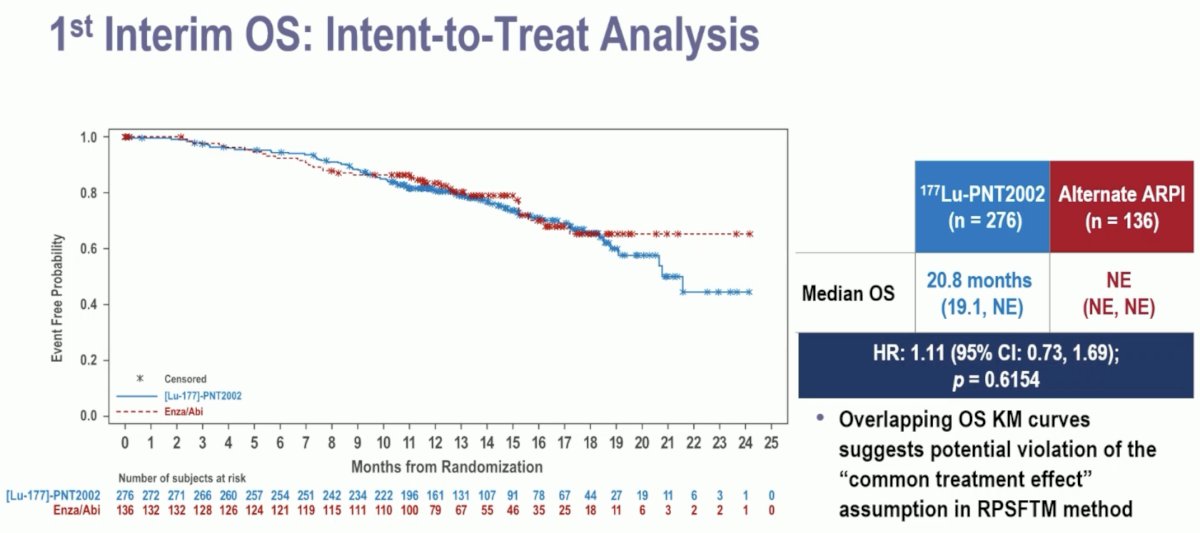
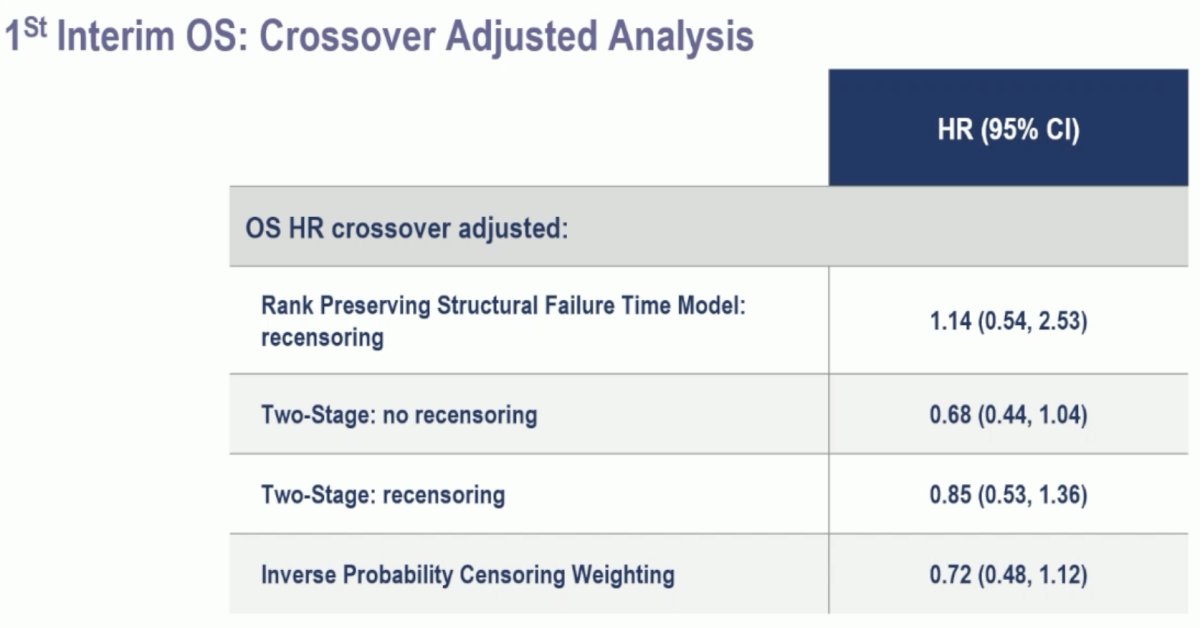
The overlapping overall survival Kaplan Meier curves suggest potential violation of the "common treatment effect" assumption in Rank Preserving Structural Failure Time Model (RPSFTM) method. Due to observed violations in assumptions for RPSFTM, other non-prespecified preliminary methods to adjust for crossover were employed resulting in overall survival HRs <1.0.
Any grade treatment-emergent adverse events were observed in nearly all patients. Overall, the adverse event profile favored 177Lu-PNT2002. Grade ≥3 events were observed in 10% and 12% of patients in the experimental and control arms, respectively. There were no treatment-related adverse events leading to death. Treatment-emergent adverse events leading to treatment discontinuation were observed in 2% and 6% of patients, respectively. Those leading to a reduction of study treatment were observed in 1% and 5%, respectively.

Grade ≥3 anemia was more common with 177Lu-PNT2002 (6% vs 3%). Arthralgia and fatigue were more common with the alternate ARPI.
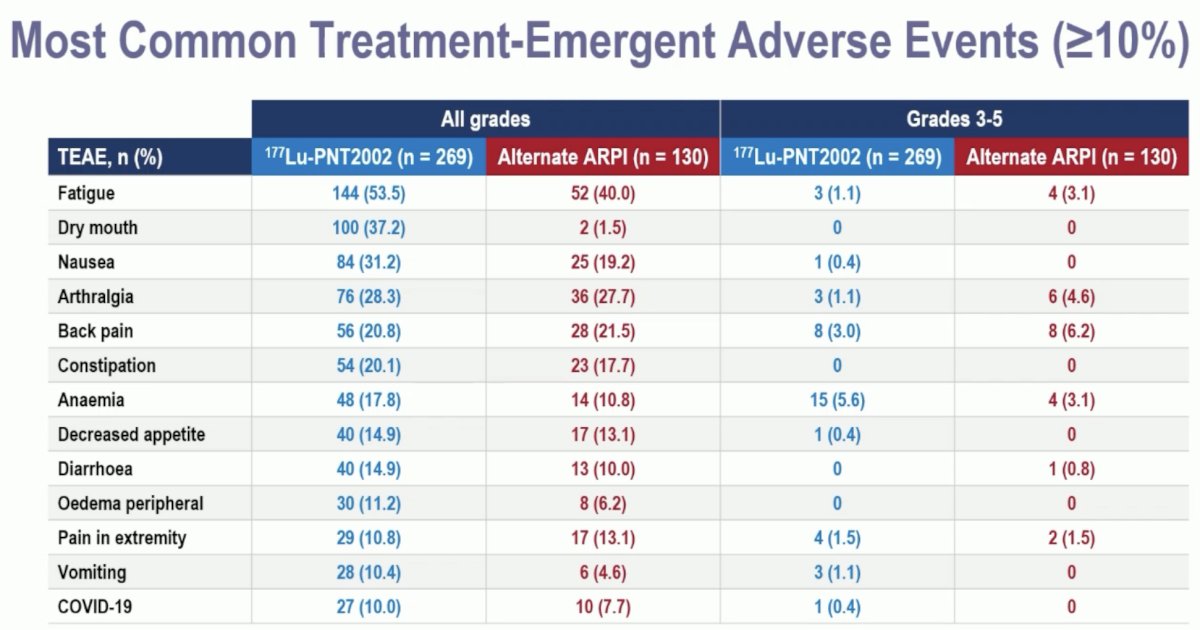
Dr. Sartor concluded as follows:
- 177Lu-PNT2002 reduced the risk of radiographic progression or death by 29% versus the use of an alternate ARPI (HR: 0.71, 95% CI: 0.55–0.92, p=0.0088)
- Overall survival data continue to mature
- Multiple secondary endpoints favor 177Lu-PNT2002, including objective response rate, PSA outcomes, and health related quality of life
- 177Lu-PNT2002’s safety profile compared favorably to the ARPI control
Presented by: A. Oliver Sartor, MD, Professor, Department of Medical Oncology, Mayo Clinic, Rochester, MN
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related Content:
SPLASH Trial Highlights Radiopharmaceutical Lutetium-177’s Role in Advanced Prostate Cancer Treatment - Oliver Sartor
ESMO 2024: Invited Discussant: Efficacy of SPLASH and UpFrontPSMA Trials


