(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a proffered paper session for prostate cancer. Professor Irene Burger provided the discussant for both SPLASH and UpFrontPSMA.
First, what are the differences between 177Lu-PSMA-617 and 177Lu-PNT2002 ([Lu 177]-PSMA-I&T)? Both molecules are very similar, with an identical PSMA-binding motive. 617 chelates a DO3A (3x COO-), while I&T has (4x COO-), therefore the overall charge for the I&T molecule is a bit more polar and thus less lipophilic. I&T has an additional iodine that can allow for dual labeling.
From a practical standpoint, however, there are no real differences between the two agents.1

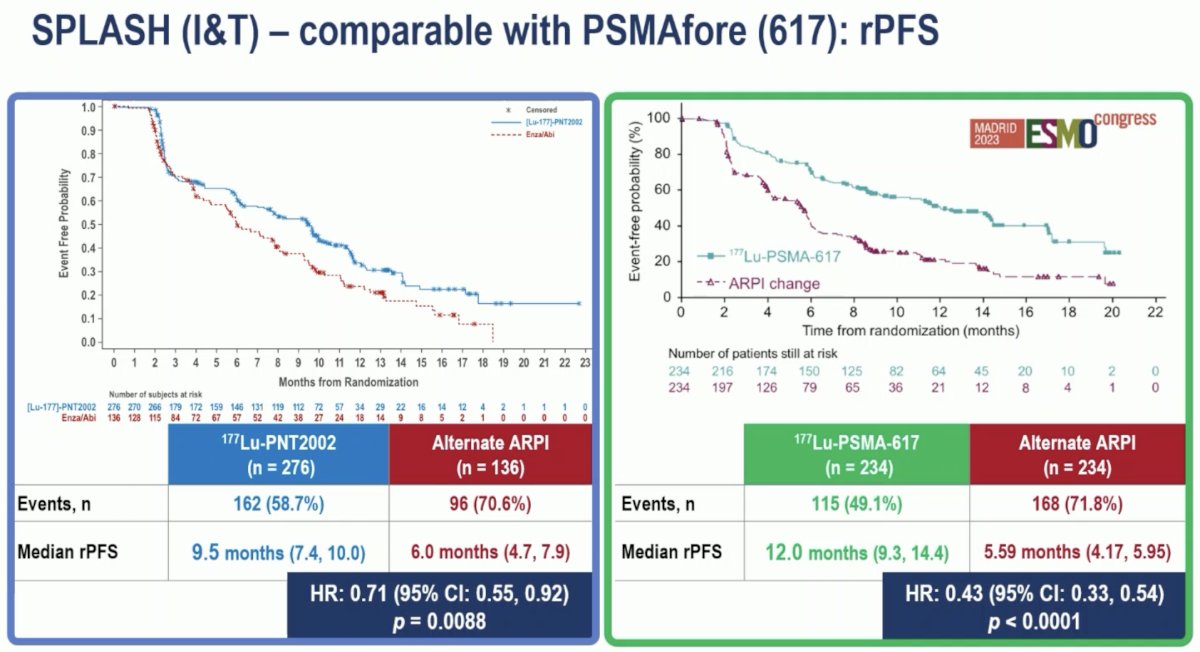
How does the study design of SPLASH compare to that of PSMAfore? Both trials enrolled patients with ARPI pre-treated, progressive mCRPC with evidence of PSMA-avid PET (no FDG PET performed). Patients were randomized in SPLASH 2:1 to 177Lu-PNT2002 ([Lu 177]-PSMA-I&T) versus an alternate ARPI. Conversely in PSMAfore, patients underwent 1:1 randomization to 177Lu-PSMA-617 versus the alternate ARPI. Notably, the radionuclide dose was significantly higher in the PSMAfore trial where patients received 7.4 GBq every 6 weeks for up to 6 cycles, versus 6.8 GBq every 8 weeks for up to 4 cycles in SPLASH. The primary endpoint in both trials was radiographic progression-free survival, assessed via blinded independent central review.
Both trials met their primary endpoint of a radiographic progression-free survival benefit, although the magnitude of benefit was greater in PSMAfore (HRs: 0.43 versus 0.71 for SPLASH).
Both trials demonstrated a health-related quality of life benefit for Lu PSMA. To date, no overall survival benefit has been observed with either study treatment. Notably, there was a high proportion of treatment crossover in both studies (84%).
From a safety standpoint, both treatments were well-tolerated. Overall, 177Lu-PNT2002 ([Lu 177]-PSMA-I&T) had a more favorable safety profile, which may be thought of as being due to the lower doses used in the SPLASH trial. However, on further assessment, Dr. Burger highlighted that even patients in the control arm of SPLASH had a more favorable safety profile, which may thus reflect the inherent patient characteristics, as opposed to the treatment received.
Dr. Berger concluded as follows with regards to the comparison of SPLASH and PSMAfore:
- 177Lu-PNT2002 reduced the risk of radiographic progression versus an ARPI switch
- When compared to PSMAfore, the lower dose and longer interval seems to reduce response (rPFS: 9.5 versus 12 months), with only some reduction in the incidence of dry mouth
- The optimal dosing and timing of Lu-PSMA therapy is still an open question.
Next, Dr. Burger moved on to discuss the UpFrontPSMA trial, shifting gears from the mCRPC to the mHSPC disease space. This trial included patients with de novo high-volume metastatic prostate cancer. All patients had high volume disease on PSMA PET, and 91% met the CHAARTED high volume criteria on conventional imaging. The lesions were predominantly PSMA-avid on PET, with no mismatch on FDG PET. Patients underwent 1:1 randomization to sequential 177Lu-PSMA-617 x 2 cycles followed by docetaxel x 6 cycles, versus docetaxel. The de novo high volume subgroup of mHSPC patients is historically that with the worst overall survival outcomes with a historic median overall survival of ~3.5 years.
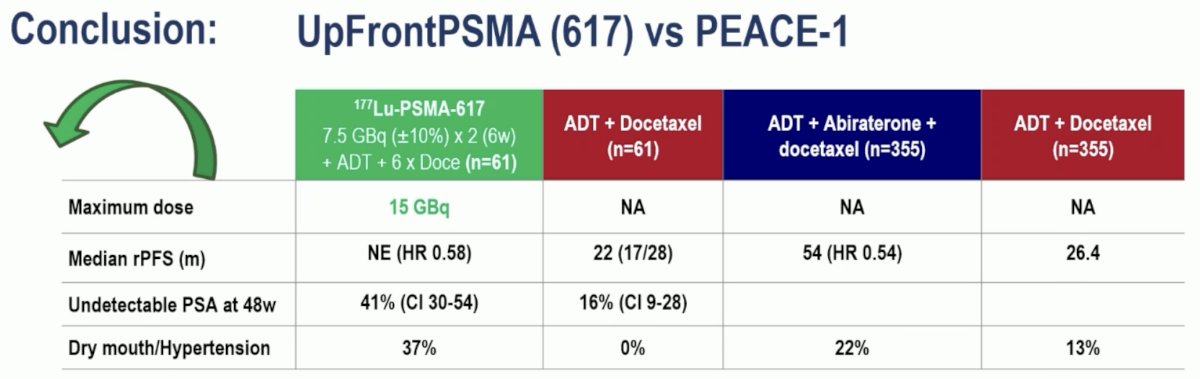
She next framed the results of UpFrontPSMA to those of PEACE-1, which also evaluated a triplet combination in de novo mHSPC patients (ADT +/- docetaxel +/- abiraterone). The radiographic progression-free survival benefit of 177Lu-PSMA-617 was similar to that of abiraterone with HRs of 0.58 and 0.54, respectively.
In contrast to PEACE-1, the Kaplan Meier curve for radiographic progression-free survival in UpFrontPSMA did not demonstrate an early separation of the survival curves. This may reflect the delayed effect of 177Lu-PSMA-617, although longer follow-up is needed.
From an adverse event standpoint, both experimental arms were well-tolerated, with comparable grade 3–4 adverse events observed across the treatment arms in both trials (UpFrontPSMA: 29% & 27% versus PEACE-1: 63% & 52%). We need to weigh the higher incidence of dry mouth adverse events with 177Lu-PSMA-617 (37% versus 0%) versus the increased incidence of hypertension with abiraterone (22% versus 13%). Importantly, the costs of both treatments (177Lu-PSMA-617 x 2 doses and abiraterone for 38 months) are roughly similar.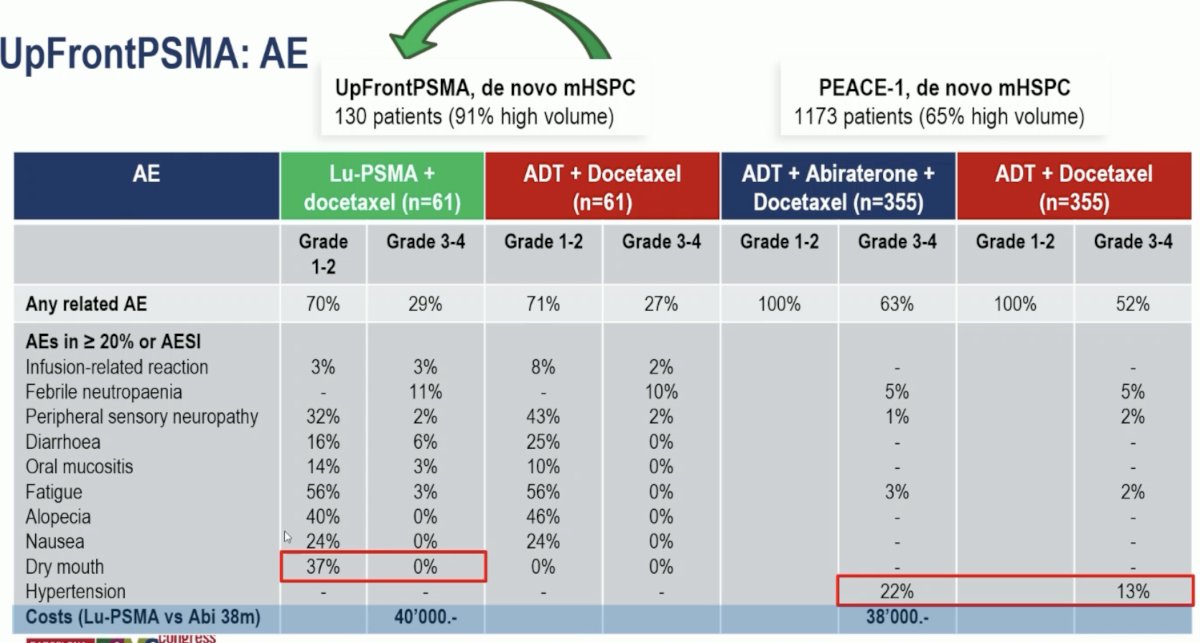
Dr. Burger concluded as follows with regards to UpFrontPSMA:
- Sequential 177Lu-PSMA-617 x2 doses reduced the risk of radiographic progression when added to ADT + docetaxel (similar to the addition of abiraterone to ADT + docetaxel)
- It has a favorable risk profile
- Radiographic progression-free and overall survivals are still preliminary
- This combination might be a good alternative to ARPI triplet therapy combination for patients with diabetes mellitus, elevated cardiac risk, etc.
She concluded this discussant section with the following remaining questions:
Presented by: Professor Irene Burger, Head of the Department of Nuclear Medicine, Kantonsspital Baden, Baden, Switzerland
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:- Schuchardt C, Zhang J, Kulkarni HR, et al. Prostate-Specific Membrane Antigen Radioligand Therapy Using 177Lu-PSMA I&T and 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: Comparison of Safety, Biodistribution, and Dosimetry. J Nucl Med. 2022; 63(8):1199-207.
Related Content:
SPLASH Trial Highlights Radiopharmaceutical Lutetium-177’s Role in Advanced Prostate Cancer Treatment - Oliver Sartor
ESMO 2024: Efficacy of 177Lu-PNT2002 in PSMA-Positive mCRPC Following Progression on an Androgen-Receptor Pathway Inhibitor (SPLASH)


