(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a proffered paper session for prostate cancer. Dr. Emily Grist presented the results of an ancillary study of the STAMPEDE Docetaxel trial evaluating the genomic classifier Decipher® mRNA score for predicting a survival benefit with docetaxel doublet therapy intensification for metastatic hormone sensitive prostate cancer (mHSPC) patients.
Dr. Grist noted that the combination of ADT + an androgen receptor pathway inhibitor (ARPI) remains a standard of care therapy for mHSPC patients.1,2 The addition of docetaxel to ADT improves survival, but at the expense of worsening quality of life.3,4
She argued that we still need validated prognostic biomarkers in this disease space, to prognosticate which patients need treatment intensification with ADT + an ARPI, as well as predictive tests to predict which cancers are docetaxel sensitive.

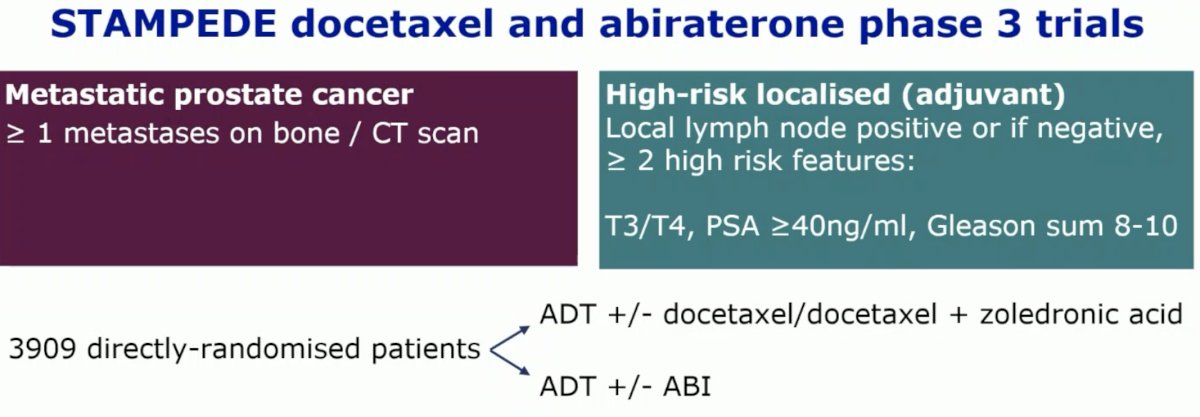
Using data from the STAMPEDE docetaxel and abiraterone phase III trials, Dr. Girst and colleagues identified 3,099 patients who were randomized to ADT +/- docetaxel +/- zoledronic acid or ADT +/- abiraterone. The aim was to link tumor multi-gene expression signatures to 14-year prospective overall survival follow-up.
Over 2,000 paraffin-fixed blocks were retrieved and then underwent central H&E review. These were subsequently microdissected and then underwent microarray analysis to identify genetic signatures. A total of 1,523 samples were deemed eligible for analysis.
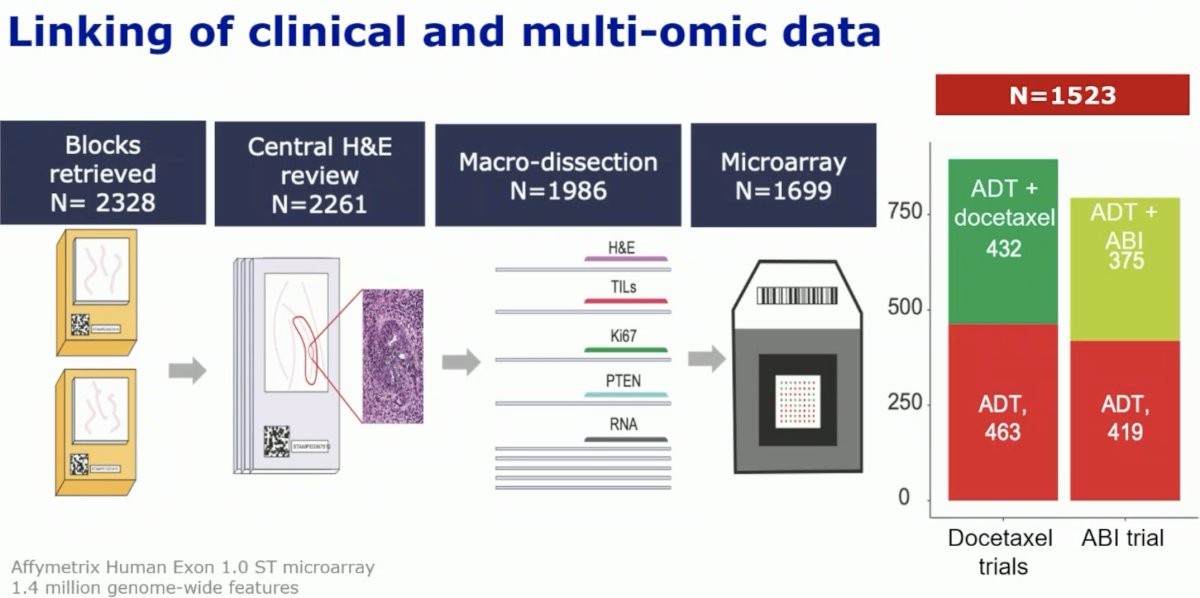
Fifty-nine signatures were derived from pan-transcriptome data. The CHAARTED biomarker cohort (n=160) was used as the training set. Four signatures were identified for predictive testing: Decipher, PAM50, PSC.
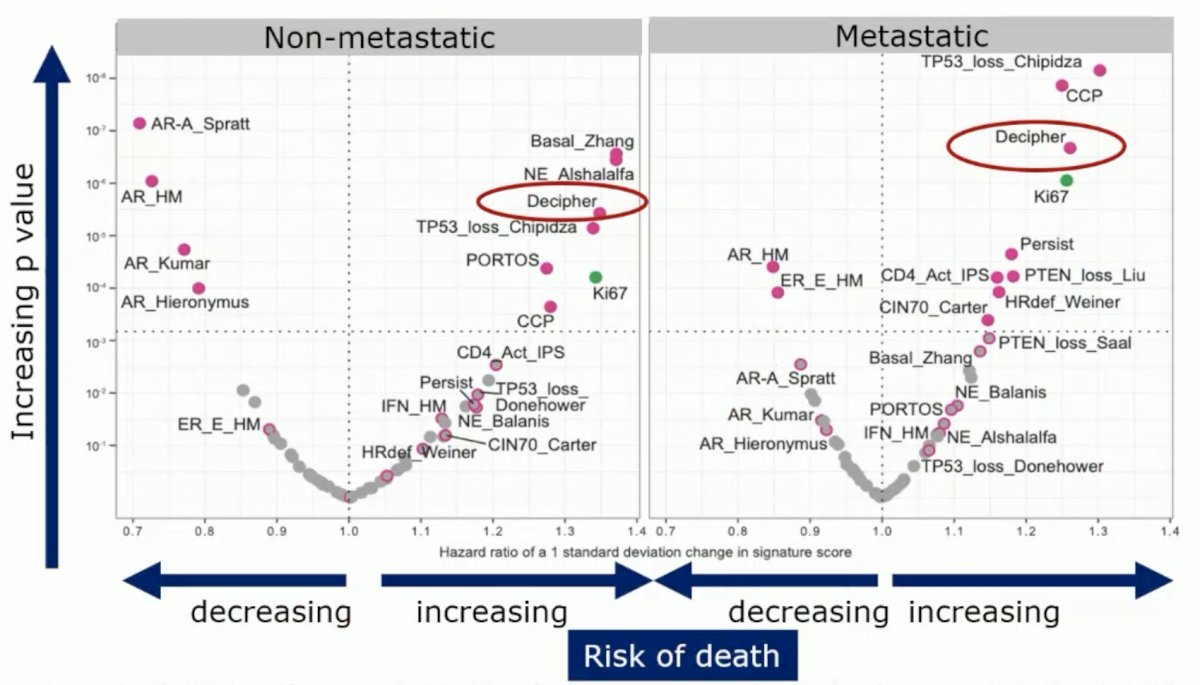
As demonstrated below, Decipher® was demonstrated to be strongly prognostic in both the non-metastatic and metastatic states. Dr. Grist noted that the androgen signaling-related signatures were protective.
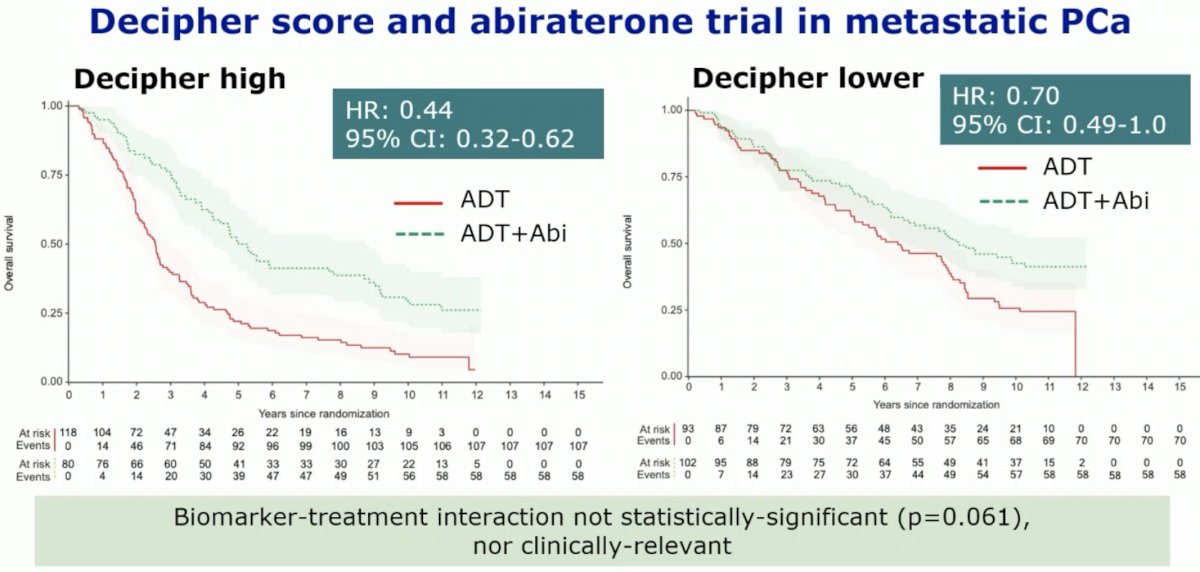
In the STAMPEDE abiraterone trial, the Decipher® score was shown to be prognostic of worse overall survival outcomes in metastatic prostate cancer patients. However, a benefit for the addition of abiraterone to ADT was observed in both Decipher® high and low score patients. The biomarker treatment interaction was neither statistically significant (p=0.061) nor clinically relevant, per the investigators’ assessment.
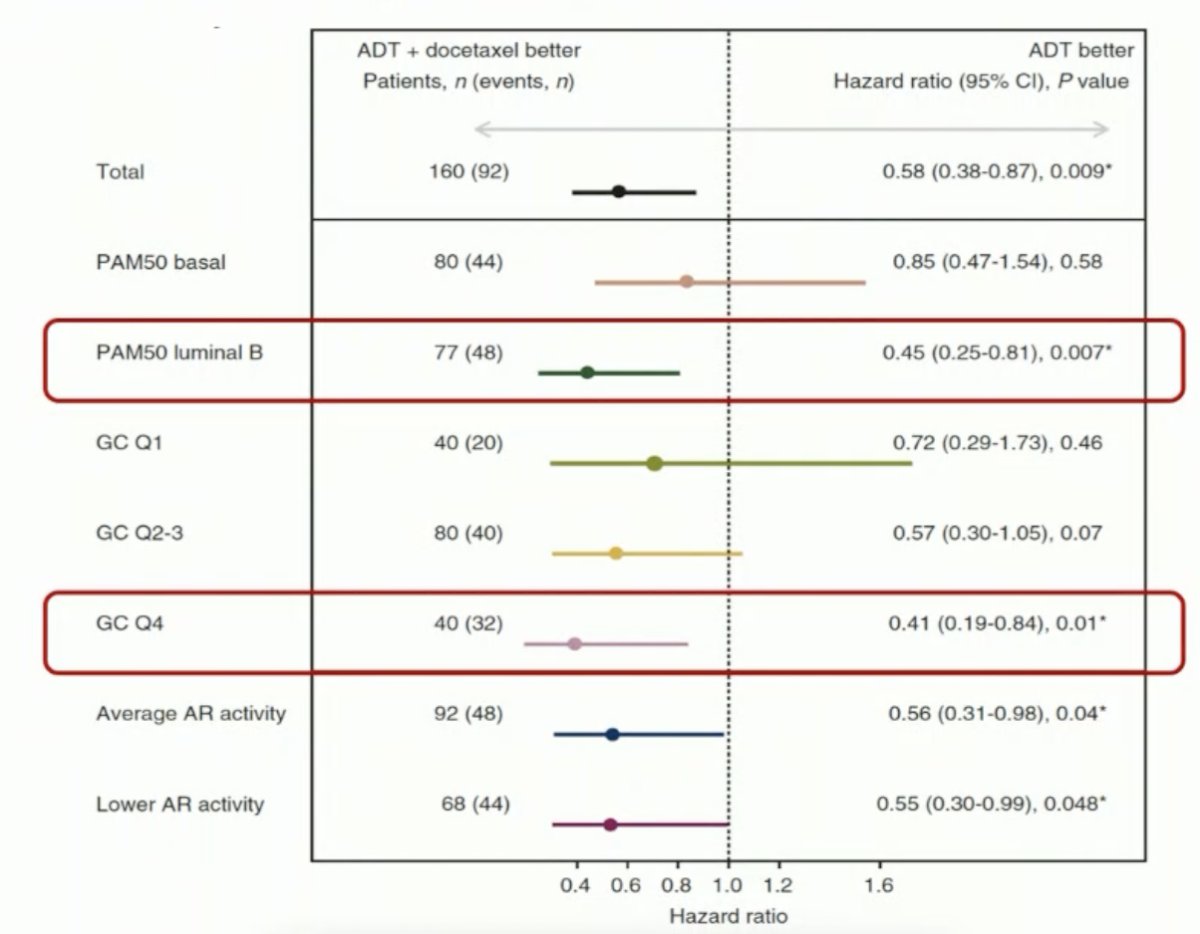
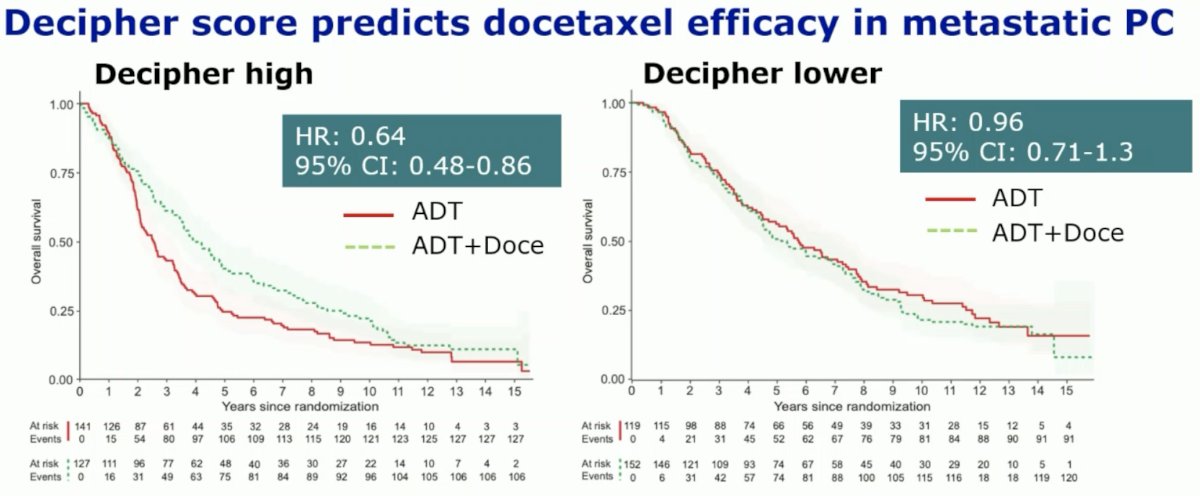
Conversely, a high Decipher® score identified patients more likely to benefit from docetaxel, with a biomarker-treatment interaction effect p-value of 0.039.
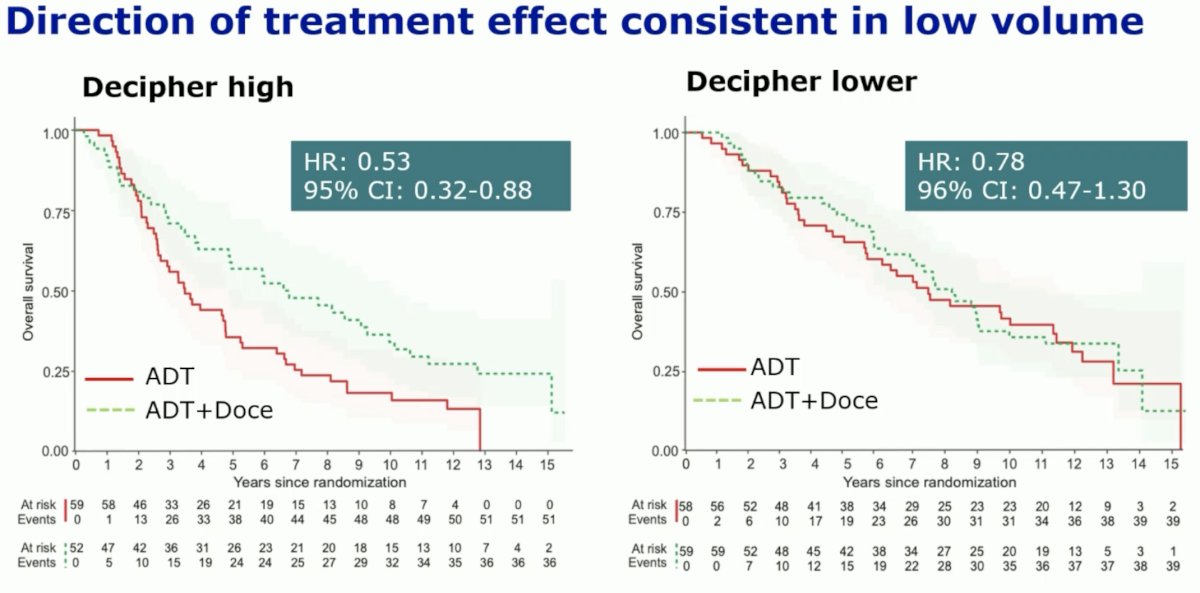
This was most notable in low volume patients, where the addition of docetaxel to ADT was associated with an overall survival benefit HR of 0.53 (95% CI: 0.32–0.88). Conversely, there was no significant overall survival benefit in the low-volume patients with lower Decipher® scores who received treatment intensification with docetaxel (HR: 0.78, 95% CI: 0.47–1.30).
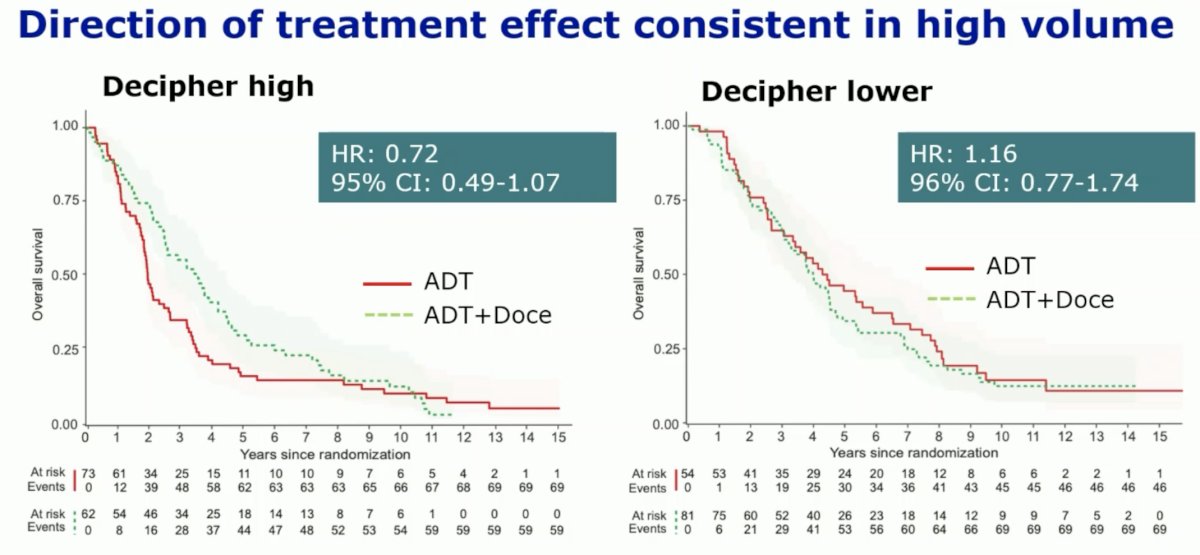
Decipher® score was similarly predictive of an overall survival treatment benefit with docetaxel addition to ADT in high volume patients.
Dr. Grist concluded as follows:
- Transcriptome signatures identify poor prognosis tumors
- High Decipher® classifier identifies patients treated with ADT + abiraterone with poor prognoses
- Decipher® classifier identifies docetaxel-sensitive tumors
- There is no clinically-significant heterogeneity of effect with abiraterone
- Decipher® classifier provides a rational biomarker for selection of patients for ADT + ARPI + docetaxel
Presented by: Emily Grist, MBBS, PhD, Cancer Institute, University College London, London, UK.
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: Decipher in mHSPC: Guiding Risk Assessment and Treatment Intensification - Gerhardt Attard
References:
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
- Rush HL, Murphy L, Morgans AK, et al. Quality of Life in Men With Prostate Cancer Randomly Allocated to Receive Docetaxel or Abiraterone in the STAMPEDE Trial. J Clin Oncol. 2022; 40(8):825-36.


