(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a proffered paper session for prostate cancer. Dr. Neeraj Agarwal presented the final overall survival results of CONTACT-02, a phase III randomized trial of cabozantinib plus atezolizumab versus a 2nd novel hormonal therapy (NHT) in patients with metastatic castrate-resistant prostate cancer (mCRPC).
mCRPC patients, especially those with visceral metastasis, who experience disease progression on NHT have a poor prognosis and limited treatment options.1,2 Although chemotherapy is an available option, a second NHT is however more frequently used in this setting (~70% vs 30%).2 As such, treatment options with novel mechanisms of action are needed.
Cabozantinib (a multi-targeted receptor tyrosine kinase inhibitor [TKI]) plus atezolizumab (anti PD-L1) have shown preliminary activity in post-NHT CRPC in a phase 1b trial.3 The combination of cabozantinib + atezolizumab versus a second NHT in mCRPC was evaluated in the phase III CONTACT-02 study (NCT04446117). The trial enrolled patients with measurable extra pelvic soft tissue metastasis who had progressed on a first NHT. CONTACT-02 met one of its dual primary endpoints of progression-free survival (HR: 0.65, 95% CI: 0.50–0.84, p=0.0007), with these results recently presented at GU ASCO 2024. In this report, Dr. Agarwal presented the final overall survival analysis, the second primary endpoint of CONTACT-02.
Patients were randomized 1:1 to cabozantinib + atezolizumab (cabozantinib [40 mg PO daily] + atezolizumab [1200 mg IV every 3 weeks]) or control (abiraterone [1000 mg PO daily] + prednisone [5 mg PO twice daily] or enzalutamide [160 mg PO daily]), and randomization was stratified by liver metastasis (yes/no), prior docetaxel for mCSPC (yes/no), and prior NHT for mCSPC, M0CRPC, or mCRPC. The trial design for CONTACT-02 is as follows: 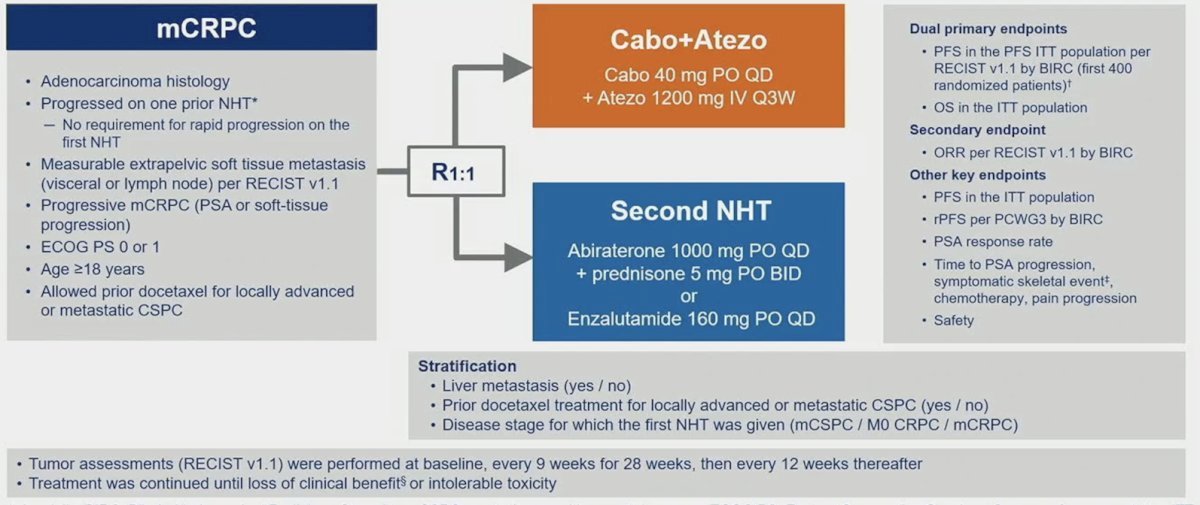
Key eligibility criteria included (i) mCRPC with disease progression on one prior NHT, (ii) measurable extrapelvic nodal or visceral disease, (iii) ECOG performance status ≤1, and (iv) ongoing ADT. Of note, docetaxel was allowed for mCSPC. The dual primary endpoints were:
- Radiographic progression-free survival by a blinded independent radiology committee (BIRC) per RECIST 1.1 in the first 400 randomized patients
- Overall survival in all randomized patients.
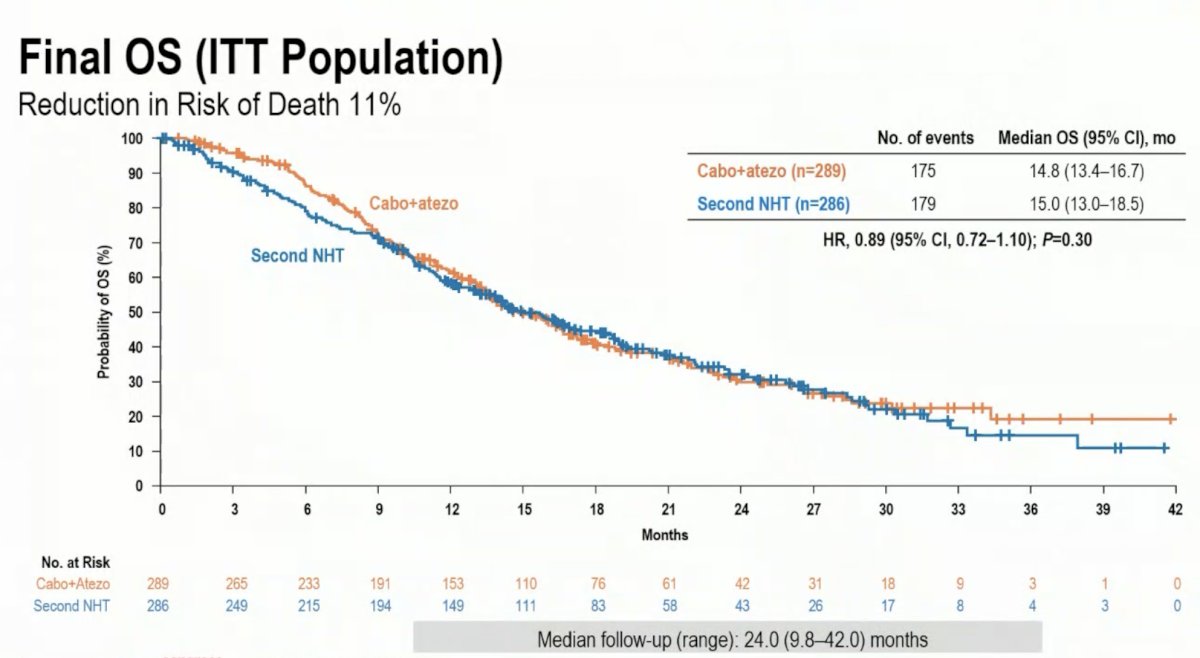
A total of 989 patients were screened, and 575 patients underwent 1:1 randomization to either cabozantinib + atezolizumab (n=289) or a second NHT (n=286). The median follow-up for the overall survival final analysis was 24 months.
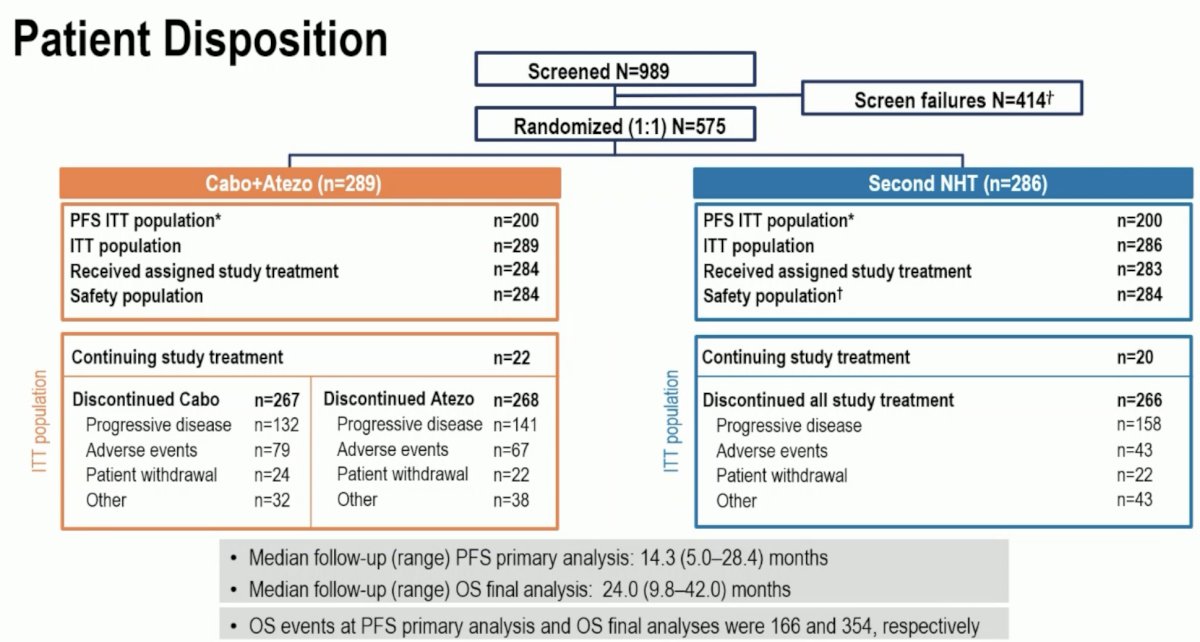
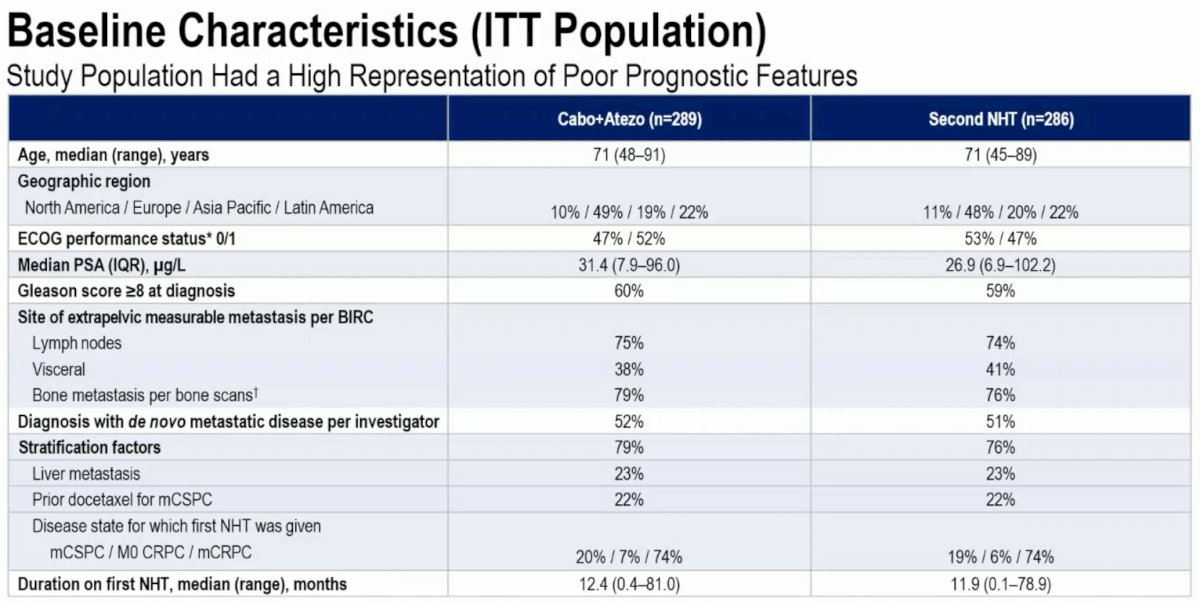
The study population had a high representation of poor prognostic features. The median PSA ranged between 27 and 31 ng/ml. 60% of patients had Gleason Score ≥8 at diagnosis. ~40% had visceral metastases, and almost 80% had bone lesions. The median duration of first NHT treatment was only 12 months.
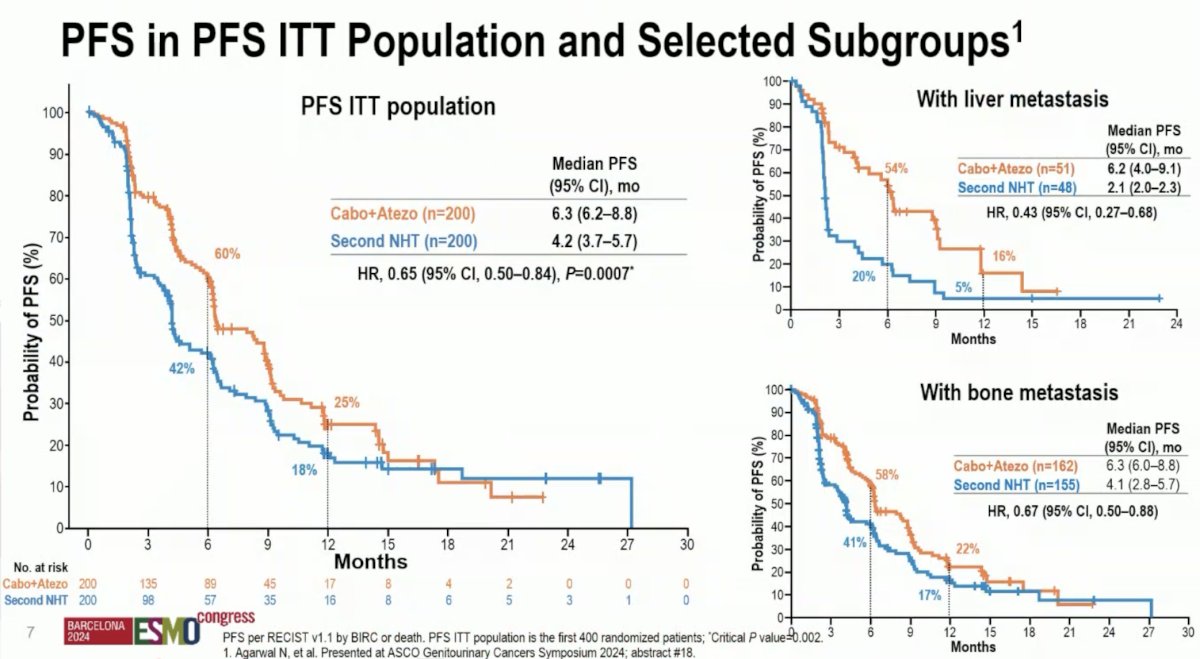
As previously presented at GU ASCO 2024, the combination of cabozantinib + atezolizumab was associated with improved progression-free survival, compared to a second NHT (median: 6.3 versus 4.2 months; HR: 0.65, 95% CI: 0.50–0.84, p=0.0007). This benefit was most notable for patients with liver metastases (median: 6.2 versus 2.1 months; HR: 0.43, 95% CI: 0.27–0.68).
The final overall survival analysis did not demonstrate a survival benefit for cabozantinib + atezolizumab (median: 14.8 versus 15 months; HR: 0.89, 95% CI: 0.72–1.10, p=0.30).
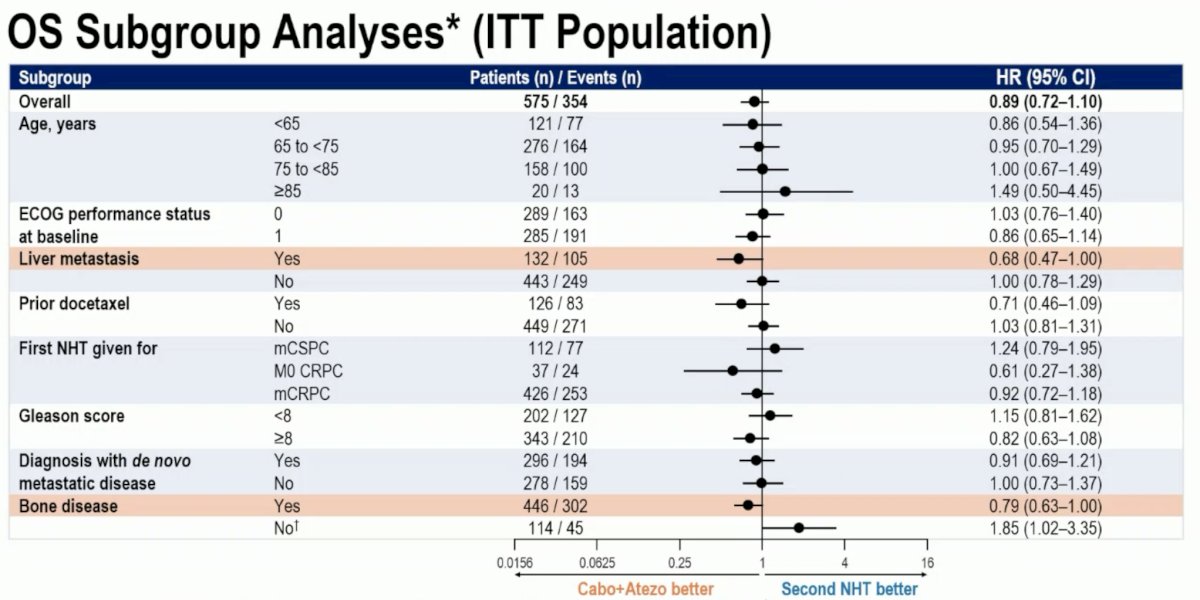
On subgroup analysis, there was suggestion of a benefit in patients with liver metastases (HR: 0.68, 95% CI: 0.47–1.00) and bone disease (HR: 0.79, 95% CI: 0.63–1.00).
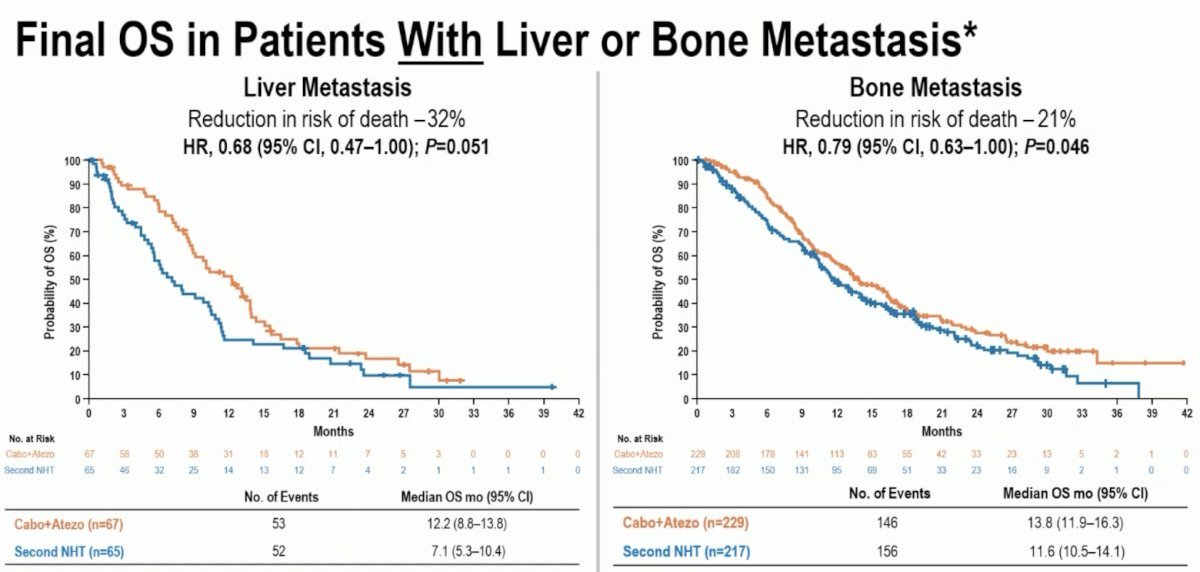
This is further represented in the Kaplan Meier curves below.
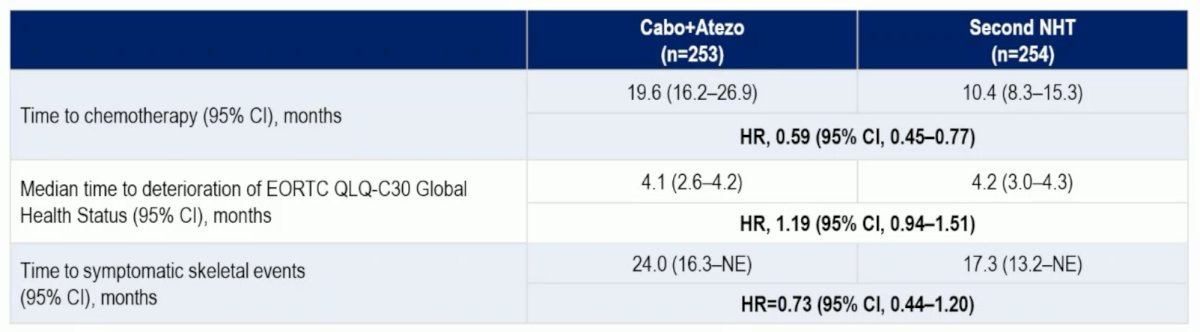
Other clinically relevant endpoints are summarized below:
With regards to adverse events, the combination of cabozantinib + atezolizumab was associated with a higher frequency of grade 3–4 adverse events (56% versus 26%). The most notable grade 3–4 events were diarrhea (5%), fatigue (6%), anemia (8%), and hypertension (8%).
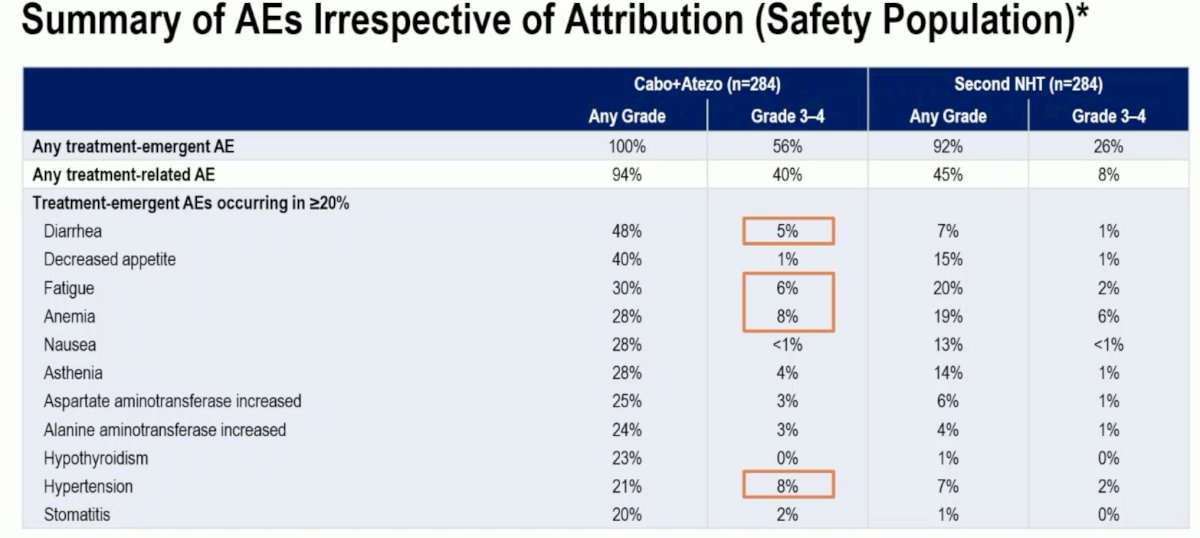
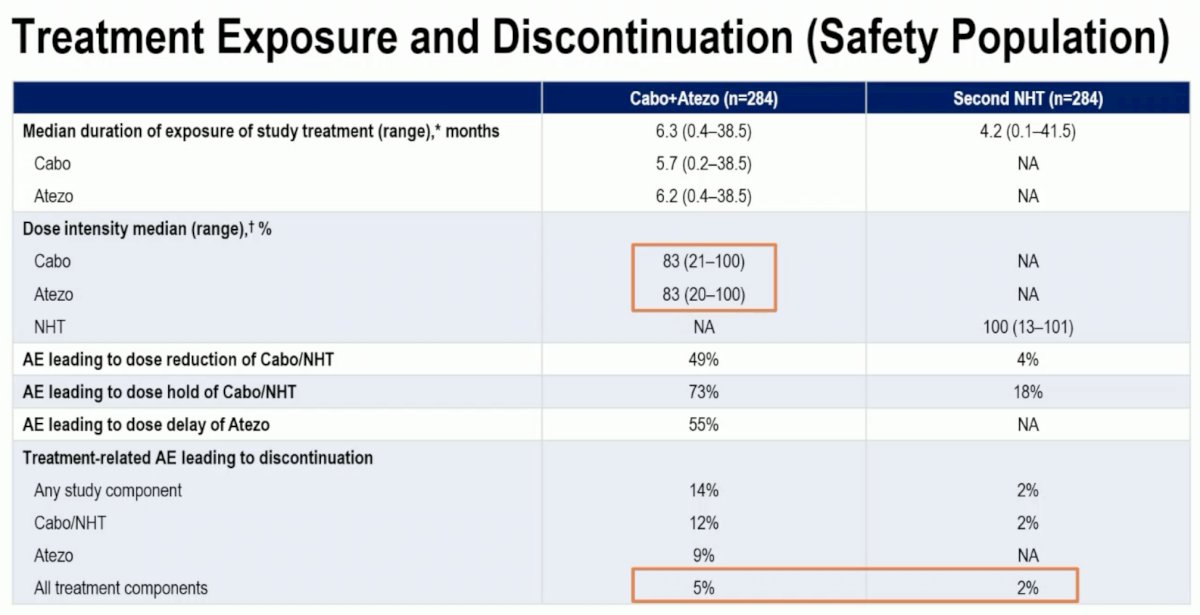
A dose reduction was required in 17% of patients in the cabozantinib + atezolizumab arm. Treatment-related adverse events leading to treatment discontinuation were observed in 5% and 2% of patients in the experimental and control arms, respectively.
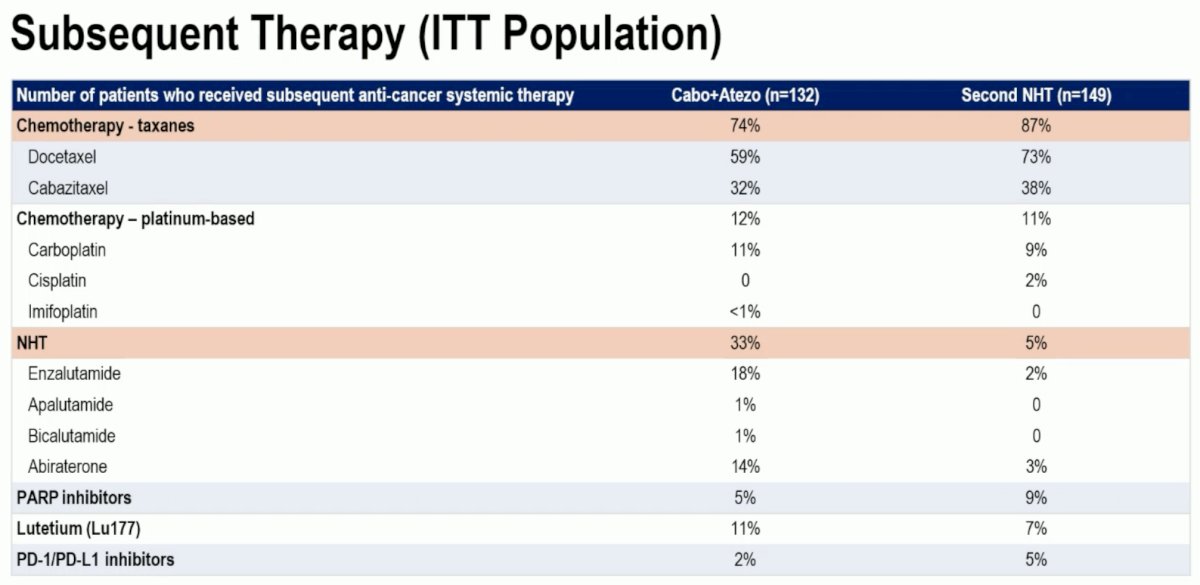
Patients in the cabozantinib + atezolizumab arm overall used taxane chemotherapy less frequency as subsequent therapy (74% versus 87%) but used NHTs more commonly in the next line setting (33% versus 5%).
Dr. Agarwal concluded as follows:
- CONTACT-02 met one of the primary endpoints, progression-free survival (HR: 0.65, p=0.0007)
- Overall survival, the second primary endpoint, favored cabozantinib + atezolizumab (HR: 0.89), but did not achieve statistical significance
- A strong survival advantage was seen in patients with liver metastasis (HR: 0.68) whose disease may be evolving to an androgen receptor undifferentiated phenotype
- Adverse events associated with the use of cabozantinib + atezolizumab were consistent with the well-established safety profile of a TKI/immune checkpoint inhibitor combination
- Cabozantinib + atezolizumab resulted in a similar median time to clinically meaningful deterioration of QoL compared to well-tolerated NHT. Times to initiation of chemotherapy and to symptomatic skeletal events were longer with cabozantinib + atezolizumab
- Cabozantinib + atezolizumab is a treatment option with a novel mechanism of action and may be useful for select patients with mCRPC who have progressed on an NHT
Presented by: Neeraj Agarwal, MD, Professor, Department of Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related coverage: CONTACT-02 Trial Results: Cabozantinib Plus Atezolizumab for mCRPC - Neeraj Agarwal
References:
- Halabi S, Kelly WK, Ma H, et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. J Clin Oncol. 2016; 34(14):1652–9.
- Swami U, Sinnott JA, Haaland B, et al. Treatment Pattern and Outcomes with Systemic Therapy in Men with Metastatic Prostate Cancer in the Real-World Patients in the United States. Cancers (Basel). 2021; 13(19):4951.
- Agarwal N, McGregor B, Maughan BL, et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: results from an expansion cohort of a multicentre, open-label, phase 1b trial (COSMIC-021). Lancet Oncol. 2022; 23(7):899-909.


