(UroToday.com) The 2024 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Ruth Langley discussing efficacy results from a randomized phase 3 evaluation of transdermal estradiol versus luteinizing hormone-releasing hormone (LHRH) agonists for androgen suppression in M0 prostate cancer. Compared to LHRH agonists, transdermal estradiol lowers testosterone more rapidly, maintains bone mineral density, and improves metabolic outcomes and quality of life. Importantly, transdermal administration avoids the cardiovascular toxicity of oral estrogen.
This was an open-label, randomized phase 3, non-inferiority comparison of LHRH agonist versus transdermal estradiol patches: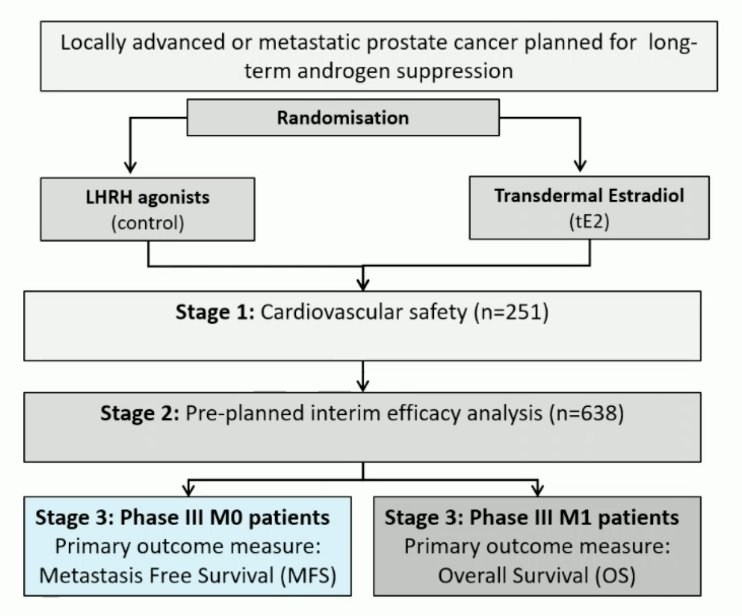
Eligibility criteria included histologically confirmed newly diagnosed high-risk M0 (locally advanced or node-positive) prostate cancer or those relapsing with PSA ≥ 4 ng/ml and doubling in <6 months, PSA ≥ 20 ng/ml, or N positive. Treatment included standard LHRH agonist versus transdermal estradiol 100 mcg/24-hour patches (four patches) changed twice weekly for ≥2 years (when testosterone <= 1.7 nmol/L, 3 patches were used and changed twice weekly); prostate radiotherapy and docetaxel were permitted. The primary outcome was metastasis-free survival, designed to rule out a >4% reduction in 3-year metastasis-free survival (85% power, 1-sided 5% α). Secondary outcomes included overall survival, castration rates, and toxicity.
There were 1,360 men, [639 LHRH agonists, 721 transdermal estradiol (randomization ratio 1:2 then 1:1)] recruited from PATCH (NCT00303784, n = 1,082) and STAMPEDE (NCT00268476, n = 278) trial sites between 2007-2022. Baseline characteristics were well-balanced between randomized groups:![There were 1,360 men, [639 LHRH agonist, 721 transdermal estradiol (randomization ratio 1:2 then 1:1)] recruited from PATCH (NCT00303784, n = 1,082) and STAMPEDE (NCT00268476, n = 278) trial sites between 2007-2022. Baseline characteristics were well-balanced between randomized groups](/images/com-doc-importer/173-esmo-2024/esmo-2024-prostate-cancer-efficacy-results-from-a-randomized-phase-3-evaluation-of-transdermal-estradiol-versus-lhrh-agonists-for-androgen-suppression-in-m0-prostate-cancer/image-1.jpg)
The LHRH agonist 3-year metastasis-free survival rate was 87% (giving a target non-inferiority HR of 1.31). Transdermal estradiol 3-year metastasis-free survival was 86% HR 0.96 (95% CI 0.81-1.14) in favor of transdermal estradiol, excluding a 2% reduction in metastasis-free survival: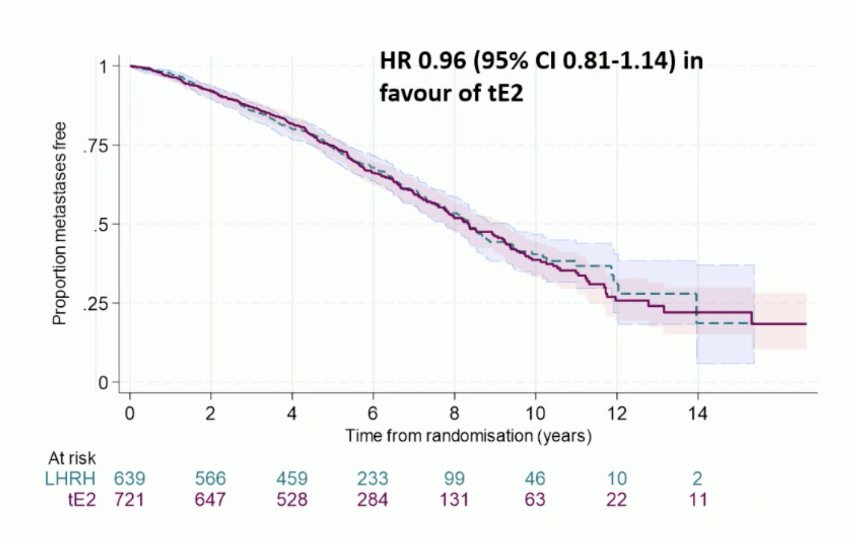
Overall survival was HR 0.89 (95% CI 0.74-1.07) in favor of transdermal estradiol: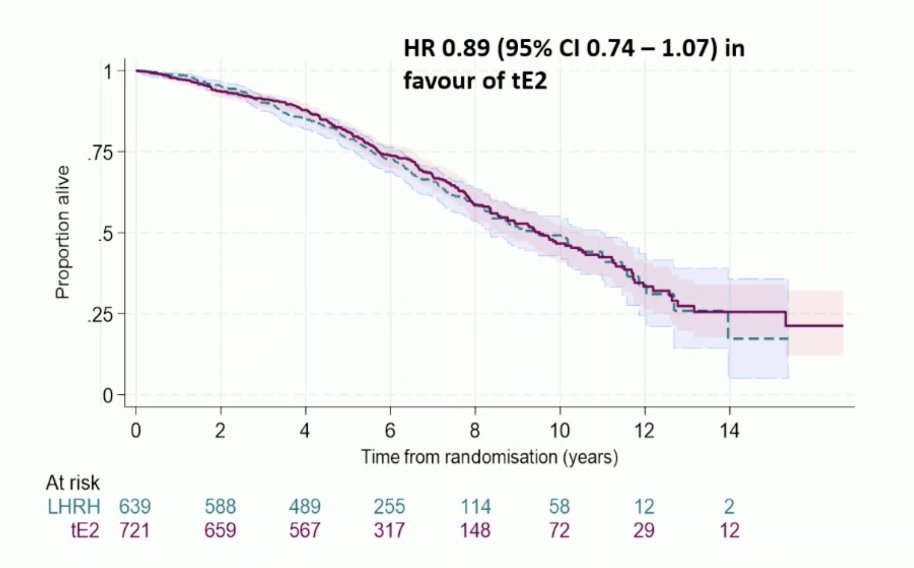
Sustained castration rate was defined as testosterone ≤1.7 nmol/L over 1 year; n = 1,066), with transdermal estradiol use confirmed as estradiol ≥250 pmol/L; 85% for both groups. LHRH agonist versus transdermal estradiol any grade adverse events included gynecomastia 42% versus 85% and hot flushes 89% versus 44%, respectively. Transdermal estradiol improved bone mineral density, there was no excess cardiovascular toxicity, and there was improved overall quality of life scores. Specifically, the mean difference in the 6-month overall score was +4.2 (1.2, 7.1) in favor of transdermal estradiol (p = 0.006).
Dr. Langley concluded her presentation discussing efficacy results from a randomized phase 3 evaluation of transdermal estradiol versus LHRH agonists for androgen suppression in M0 prostate cancer with the following take-home points:
- Transdermal estradiol is as effective as LHRH agonist and there is no detriment in terms of prostate cancer outcomes or overall survival in starting androgen suppression with transdermal estradiol
- Transdermal estradiol provides choice about expected side effects and route of administration allowing for personalized treatment plans.
- Transdermal estradiol should be a standard-of-care ADT option in M0 disease.
Presented by: Ruth E. Langley, PhD, Professor, MRC Clinical Trials Unit at UCL, London, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: PATCH Trial Evaluates Transdermal Estradiol in Non-Metastatic Prostate Cancer - Duncan Gilbert


