(UroToday.com) The 2024 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Niven Mehra discussing nivolumab 3mg/kg and ipilimumab 1mg/kg in molecularly selected patients with mCRPC. Anti-PD1 immune checkpoint blockade is currently approved as monotherapy for mCRPC patients with a tumor mutational burden (TMB) of >10 mutations per megabase (mut/Mb) or mismatch repair deficiency.
Dual anti-PD1/CTLA-4 appears more effective than monotherapy, but the wide applicability of nivolumab 1mg/kg/ipilimumab 3mg/kg in mCRPC is constrained by its toxicity. This study investigates the efficacy and safety of nivolumab 3mg/kg and ipilimumab 1mg/kg in molecularly selected mCRPC, representing ~15% of patients with mCRPC.
INSPIRE is a single arm, phase II trial, including 69 molecularly selected mCRPC patients with mismatch repair deficiency, non-synonymous TMB >7 mut/Mb (hTMB), a BRCA2 mutation, or biallelic inactivation of CDK12 (CDK12i). Efficacy was evaluated in cohort A, which included immune checkpoint blockade-naïve patients with RECIST1.1 (A1) and PCWG3 (A2) measurable disease. Safety was evaluated in cohorts A and B (prior immune checkpoint blockade monotherapy). Patients were treated with nivolumab 3mg/kg and ipilimumab 1mg/kg Q3W for 4 cycles, followed by nivolumab 480 mg Q4W up to 1 year. The primary endpoint was disease control rate > 6 months, aiming to surpass a disease control rate > 6 months of 22%. The trial design for INSPIRE is as follows:
The biomarker and screening program and CONSORT diagram for INSPIRE are highlighted below: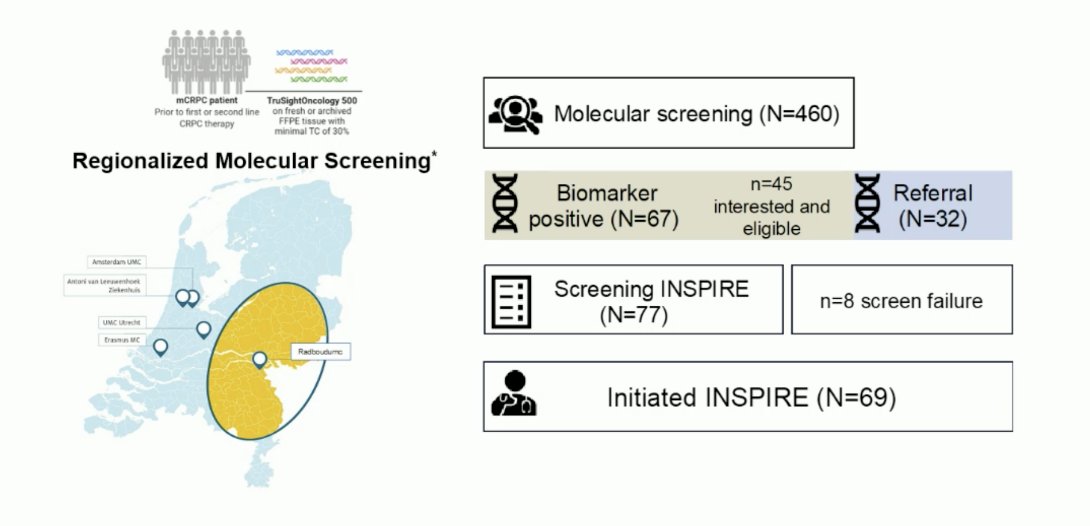
Patients initiated treatment between January 2021 and February 2024. The median age was 69 [range 50-82]. Cohort A consisted of 65 patients, 21 patients had mismatch repair deficiency (32%), 8 hTMB (12%), 20 BRCA2 mutations (31%), and 16 CDK12i (25%):![The median age was 69 [range 50-82]. Cohort A consisted of 65 patients, 21 patients had mismatch repair deficiency (32%), 8 hTMB (12%), 20 BRCA2 mutations (31%) and 16 CDK12i (25%)](/images/com-doc-importer/173-esmo-2024/esmo-2024-inspire-phase-2-trial-of-nivolumab-3mg-kg-and-ipilimumab-1mg-kg-in-molecularly-selected-patients-with-mcrpc/image-2.jpg)
The median number of prior mCRPC treatments was 1 (range 0-5), 68% had received prior taxane chemotherapy, 77% had received prior androgen receptor pathway inhibitors, and the most common site of disease was bone metastasis (>80%):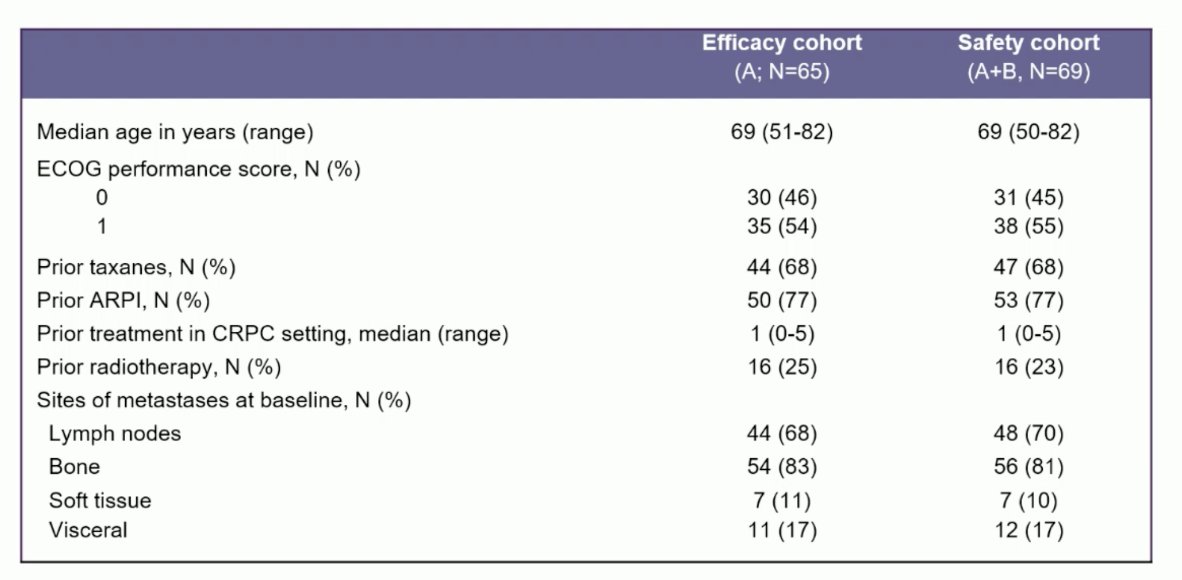
At the data cut-off, the median follow-up was 12 months (range 1-42). Disease control rate > 6 months was reached in 38% (95% CI 27-51) of patients and was highest in mismatch repair deficiency patients (81%), followed by hTMB (25%), CDK12i (19%) and BRCA mutated patients (15%). In the overall cohort, objective response rate, PSA50, and PSA90 responses were 38%, 47%, and 41%, respectively. The median radiographic progression-free survival was 4.0 months (95% CI, 3.5 to 12.0) in cohort A and 32.7 months in mismatch repair deficiency patients (95% CI, 21.8 to NR):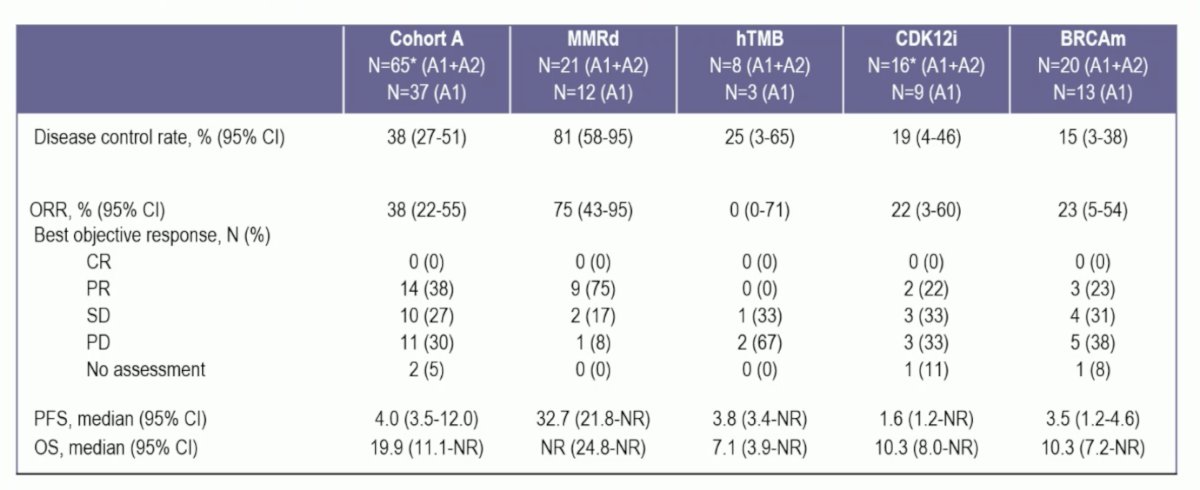
The Kaplan-Meier curves for progression-free and overall survival are highlighted: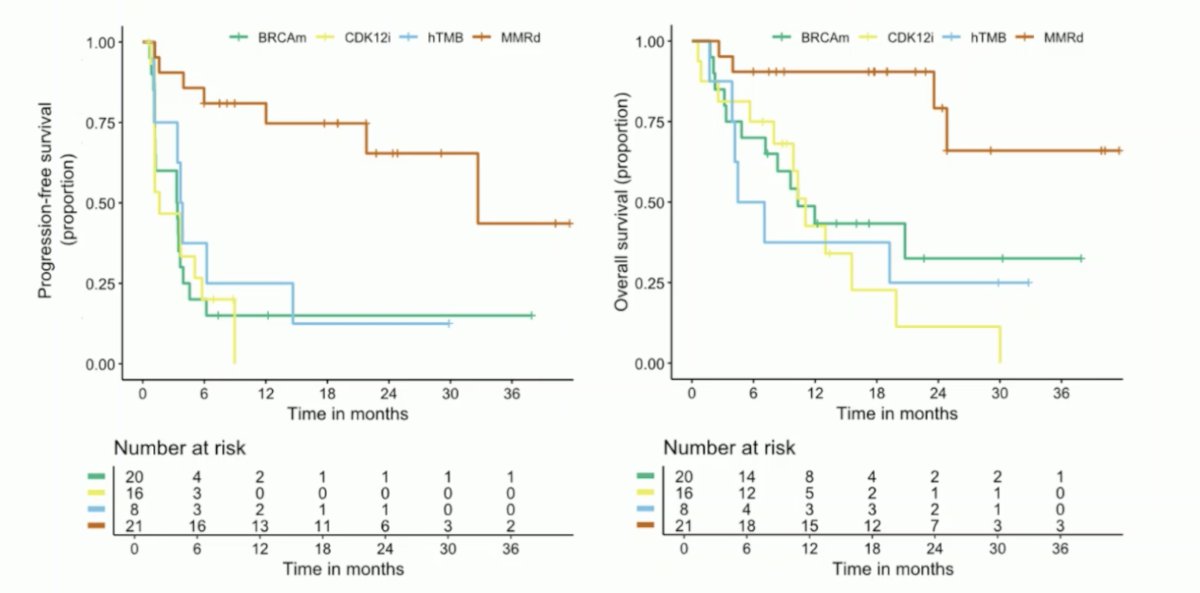
Treatment-related adverse events led to permanent discontinuation in 14 patients (20%), and grade ≥3 treatment-related adverse events occurred in 33 patients (48%), including diarrhea and elevated transaminases (each in 10% of patients). There were 2 treatment-related deaths, including. a bowel perforation and euthanasia following grade 4 toxicity: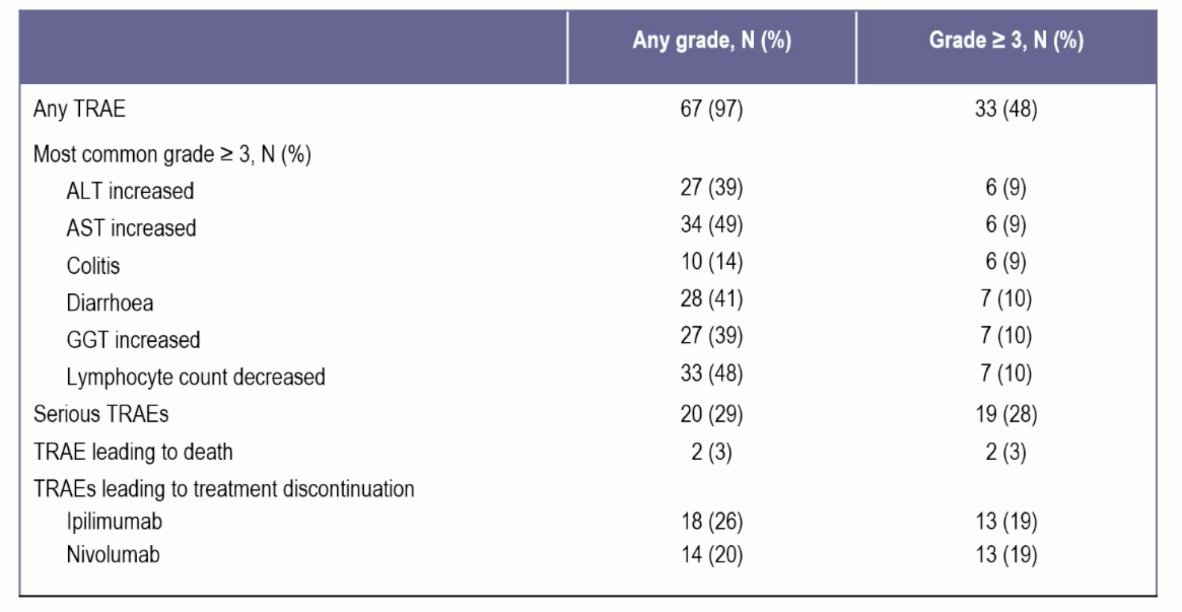
Dr. Mehra concluded his presentation discussing nivolumab 3mg/kg and ipilimumab 1mg/kg in molecularly selected patients with mCRPC with the following take-home points:
- This phase II trial evaluated the efficacy and safety of nivolumab 3 mg/kg + ipilimumab 1 mg/kg in molecular-selected mCRPC patients, including me with mismatch repair deficient, hTMB, BRCA mutations, and CDK12i
- The study met the primary endpoint, demonstrating a > 6-month disease control rate of 38%
- There was limited efficacy seen in hTMB, CDK12i, and BRCA mutation cohorts
- Responses were robust in patients with mismatch repair deficiency, demonstrating a > 6 months disease control rate of 81%, objective response rate of 75%, PSA90 response rate of 86%, and a median progression-free survival of 32.7 months.
- These findings underscore the need for early testing and treatment of mismatch repair deficient mCRPC with dual immune checkpoint inhibitors.
- Future research is still necessary, given that ~5% of metastatic prostate cancer patients harbor mismatch repair deficiency, therefore, international collaborative efforts are necessary to further develop precision medicine in mismatch repair deficient prostate cancer.
Presented by: Niven Mehra, MD, Radboud University Medical Center, Nijmegen, Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


