(UroToday.com) The 2024 ESMO annual meeting included a session on prostate cancer, featuring a discussant presentation by Dr. Deborah Mukherji discussing two abstracts including “RAPSON: Open-label, multicenter randomized trial of Radium-223 -> docetaxel versus docetaxel -> Radium-223 sequence in mCRPC with prospective biomarker evaluation” by Dr. Vincenza Conteduca and “Adding metformin to ADT for patients with mHSPC: Overall survival results from the multi-arm, multi-stage randomized platform trial STAMPEDE” by Dr. Silke Gillessen.
The RAPSON study was a prospective, multicenter, randomized phase 2 trial in 70 patients with symptomatic bone-dominant mCRPC who progressed after ADT ± abiraterone or enzalutamide. Patients were initially randomized 1:1 to receive Radium-223 or docetaxel as first treatment followed by docetaxel or Radium-223, respectively, at progression. The trial design for RAPSON is as follows:

Dr. Mukherji noted that the primary endpoint was the impact of sequential therapy on the percentage of patients with symptomatic bone-dominant mCRPC experiencing changes in health-related quality of life measured with the Functional Assessment of Cancer Therapy Prostate (FACT-P) questionnaire from baseline to week 12. At a median follow-up of 13 months (range 4-45), a preliminary responder analysis at week 12 showed 33% versus 50% of patients experiencing a worsening of FACT-P questionnaire score (22% versus 50% in the physical well-being subscale) in Radium-223 versus docetaxel arm, respectively:
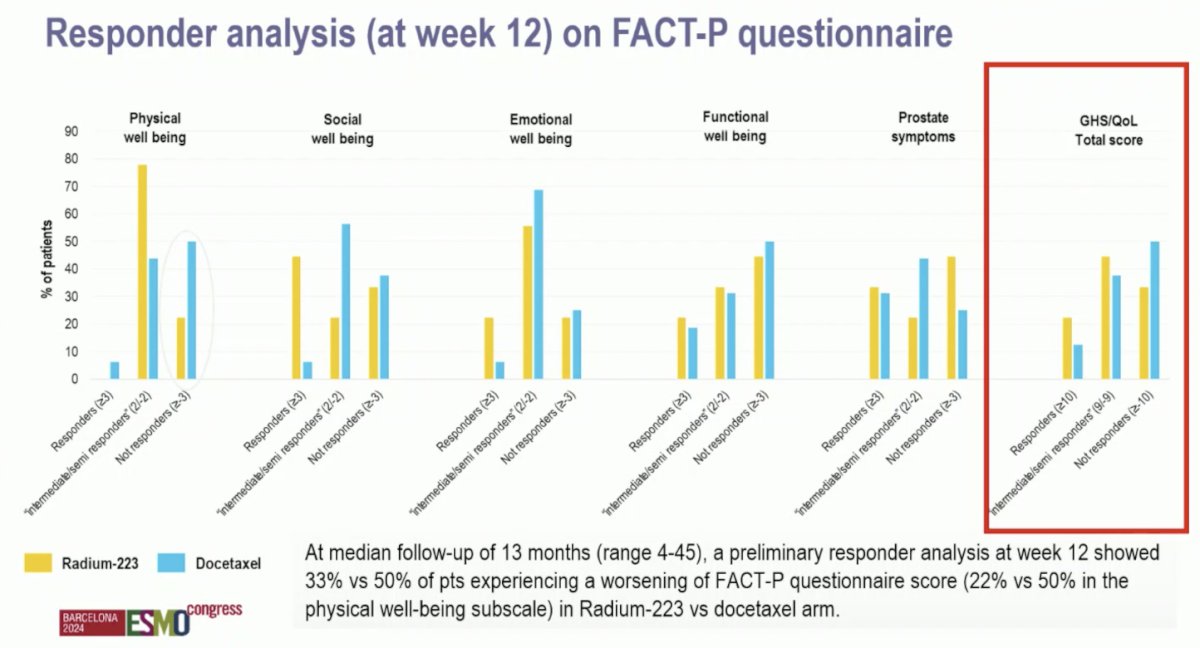
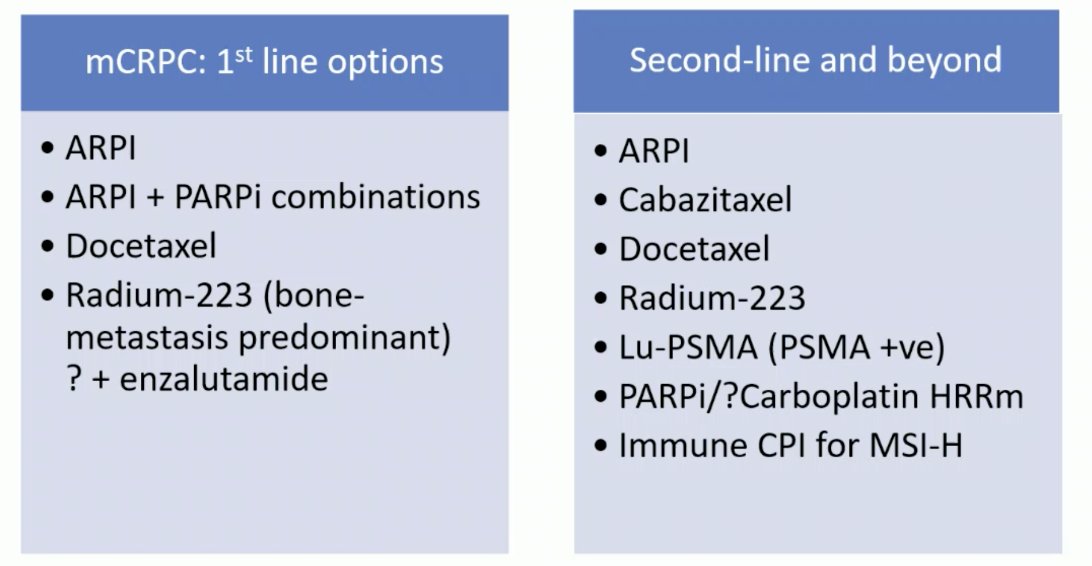
Dr. Mukherji emphasized that for patients and their priorities with regards to treatment sequencing and quality of life: the main goal is living as well as possible for as long as possible. In RAPSON, there was no significant difference in progression free survival and overall survival observed between the two sequence arms: Arm A (Radium-223 docetaxel) vs Arm B (docetaxel Radium-223). Currently, we have a plethora of CRCP treatment options both in the first line and second line settings:
At ESMO 2024, we now have new data supporting even earlier use of radiopharmaceuticals in prostate cancer:’
- PEACE-3: Radium-223 + enzalutamide
- SPLASH: 177Lu-PNT2002
- UpFrontPSMA: 177Lu-PSMA-617 in mHSPC
Furthermore, there was the recent Lancet publication during ESMO 2024 of PSMAfore, which improved radiographic progression free survival and improved quality of life in taxane-naïve mCRPC patients.1 Dr. Mukherji provided the following concluding statements regarding the RAPSON study:
- Patients with symptomatic bone-predominant mCRPC had overall better quality of life scores at 12 weeks on Radium-223 versus docetaxel, with no significant difference in progression free and overall survival between the two arms so far
- This aligns with patient priorities for research into treatment sequencing and focusing on quality of life
- The primary endpoint was based on an early quality of life assessment (4 cycles of docetaxel or 3 cycles of Radium-223), so it is not surprising that patients on docetaxel did not feel as well after 12 weeks
- This adds to the growing evidence to support giving Radium-223 (and other radiopharmaceuticals) earlier in the patient treatment journey, however regulatory approvals and access vary globally
Dr. Mukherji then discussed the STAMPEDE metformin trial among patients with mHSPC. Non-diabetic patients with mHSPC were randomly allocated 1:1 to standard of care or standard of care + metformin within STAMPEDE. Standard of care included ADT ± radiotherapy ± docetaxel ± androgen receptor pathway inhibitor. The primary outcome was overall survival. Dr. Mukherji made note of (i) enrolled patients having a HbA1c <6.5% and no treatment for diabetes, (ii) 83% of patients in the standard of care arms receiving ADT + docetaxel, and (iii) a lofty goal of 20% improvement in overall survival:

After a median follow-up of 60 months, the HR for overall survival between arms was 0.91 (95% CI 0.80-1.03; p = 0.148). The median overall survival was 63.1 (95% CI 57.6-68.7) and 69.1 (95% CI 62.7-73.2) months in the standard of care and standard of care + metformin arms, respectively. However, in patients with high versus low volume disease (CHAARTED definition), HR was 0.79 (95% CI 0.66-0.93; p = 0.006) and 1.00 (95% CI 0.79-1.26; p = 0.992), respectively. The potential benefit in high volume patients was also corroborated when looking at progression free survival (HR 0.76, 95% CI 0.64-0.89):
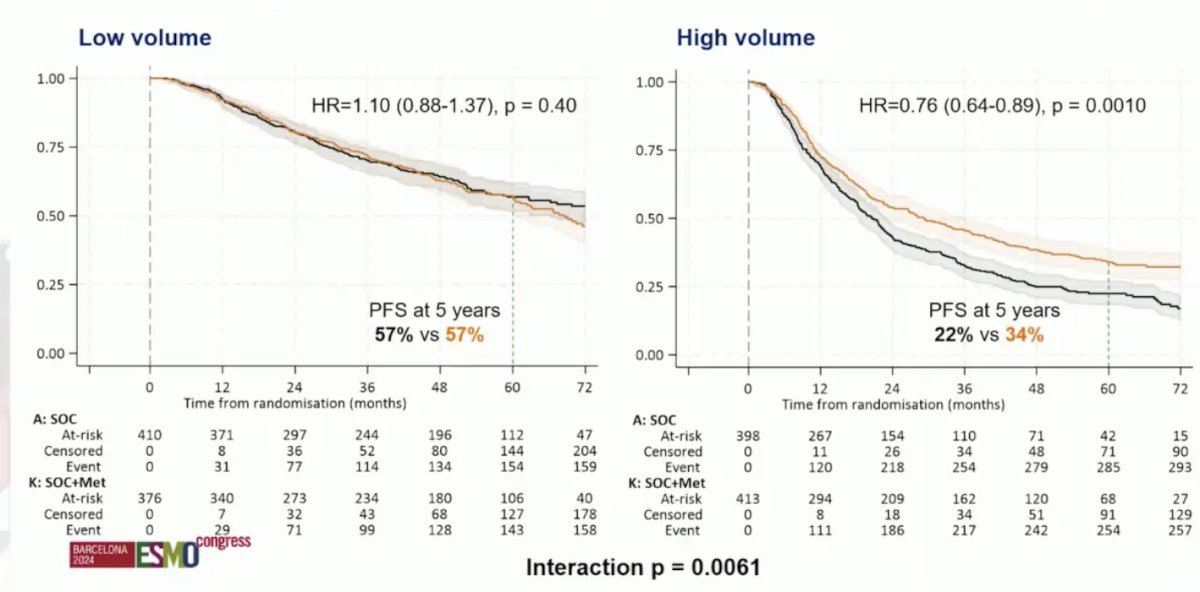
Could metformin help control biologically aggressive disease? There is a suggestion of AMPK-dependent and independent mechanisms, but the challenge is identifying predictive biomarkers. Based on The Lancet’s Global Commission on Prostate Cancer, there is an estimated doubling of new cases of prostate cancer globally by 2040 to 2.9 million cases annually. Importantly, the largest increase is in lower resource settings with high rates of advanced disease at diagnosis. Moreover, there is an increasing prevalence of other non-communicable diseases including cardiovascular disease and diabetes. In Korea, among 100,000 men diagnosed with prostate cancer, >50% of men were taking metformin at the time of their diagnosis, which demonstrated improved overall and cancer specific survival. One limitation is that there was no information on stage at diagnosis and no information on treatment received. The MANSMED trial was a randomized trial from Egypt in high risk/M1 prostate cancer randomizing 124 men to standard of care + metformin versus standard of care alone:2
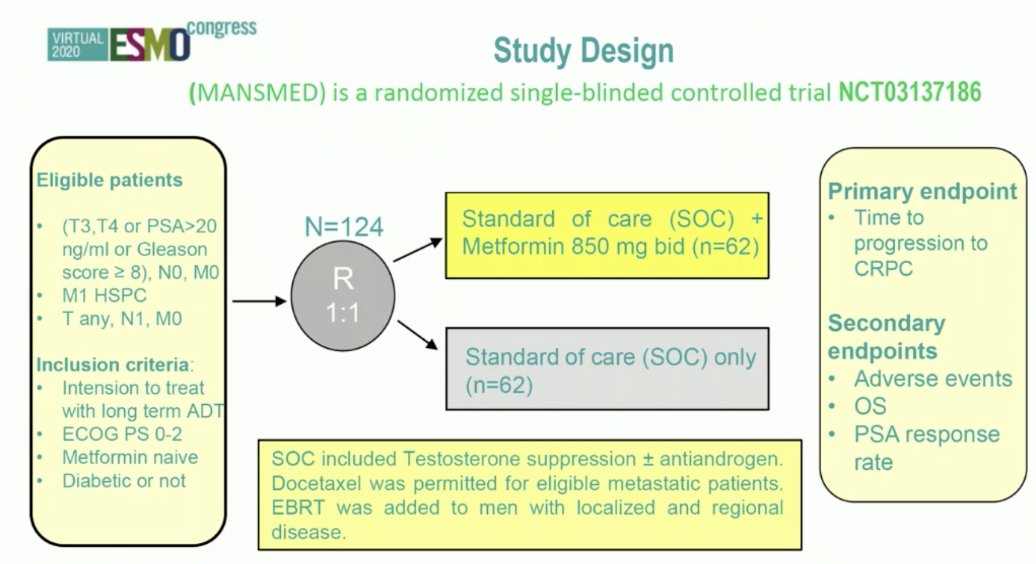
MANSMED showed that men in the standard of care + metformin arm had a median time to CRPC of 29 months versus 20 months in the standard of care arm. Dr. Mukherji provided the following concluding statements regarding the STAMPEDE Metformin trial:
- In non-diabetic patients with mHSPC, adding metformin to standard of care (>80% ADT + docetaxel) did not improve overall survival
- This was a high bar to set for an inexpensive well-tolerated treatment
- Metabolic parameters improved, however quality of life/cardiovascular events were not presented and will be important data to follow
- Improved overall survival and progression free survival in high volume subgroups (pre-specified but not pre-powered) is not enough evidence to recommend the addition of metformin for non-diabetic men with prostate cancer
- But, in the context of a rising incidence of prostate cancer and other non-communicable diseases globally, further studies on the long-term benefits of glycemic control and other effects of metformin in men with advanced prostate cancer are warranted
Presented by: Deborah Mukherji, MBBS, FRCP, Clemenceau Medical Center Dubai, Dubai, United Arab Emirates
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Morris MJ, Castellano D, Herrmann K, et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naïve patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomized, controlled trial. Lancet 15 Sept 2024 [Epub ahead of print].
- Alghandour R, Ebrahim MA, Eishal AM et al. Repurposing metformin as an anticancer drug: Randomized controlled trial in advanced prostate cancer (MANSMED). Urol Oncol. 2021 Dec;39(12):831.e1-831.e10.


