(UroToday.com) The 2024 ESMO annual meeting included a session on prostate cancer, featuring a discussant presentation by Dr. Niven Mehra discussing three abstracts including “Efficacy and safety of darolutamide plus ADT in patients with mHSPC from the phase 3 ARANOTE trial” by Dr. Fred Saad, “Prostate cancer efficacy results from a randomized phase 3 evaluation of transdermal estradiol versus LHRH agonists for androgen suppression in M0 prostate cancer” by Dr. Ruth Langley, and “Phenotypic and genomic characterization of de novo metastatic prostate cancer: an ancillary study of the PEACE-1 phase 3 trial” by Dr. Cedric Pobel.
Starting with the phase 3 ARANOTE trial, Dr. Mehra notes that ARANOTE is the fourth trial to the disease space of ARPI + ADT versus Placebo + ADT for mHSPC. For ARANOTE, eligible patients had mHSPC by conventional imaging, an ECOG performance status of 0–2, and started ADT ≤ 12 weeks. Patients were randomized 2:1 to darolutamide 600 mg twice daily or placebo, each with ADT. The primary endpoint was radiological progression-free survival and the trial design for ARANOTE is below:
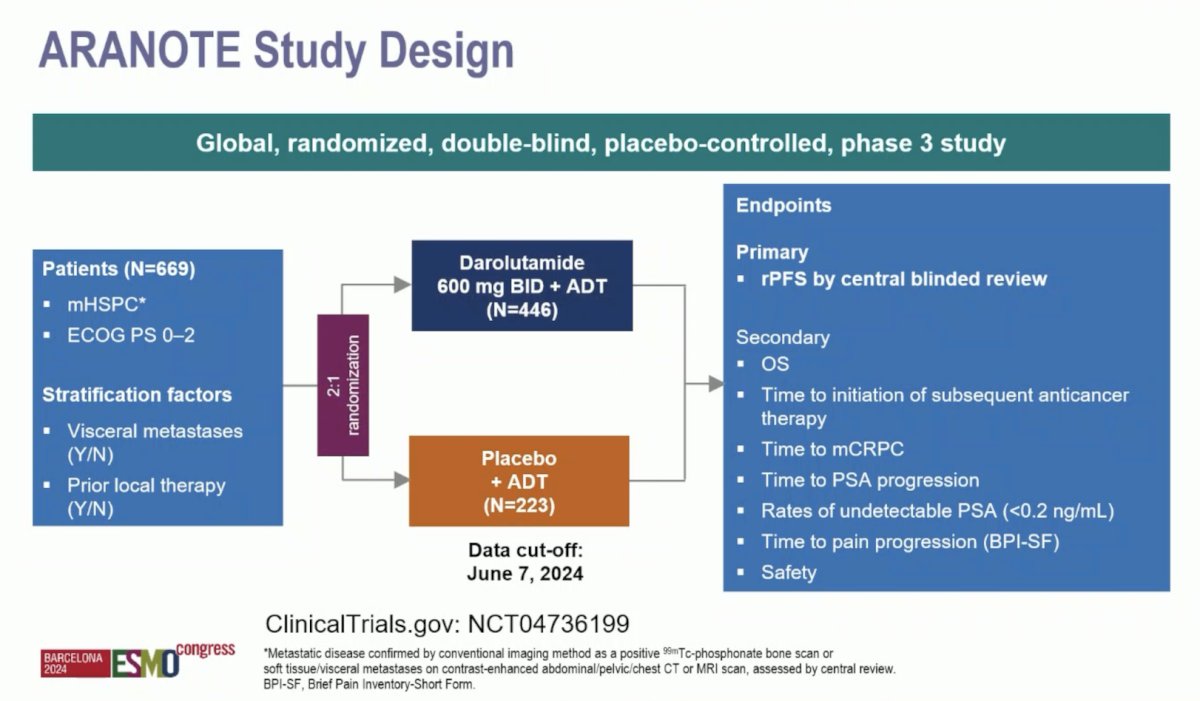
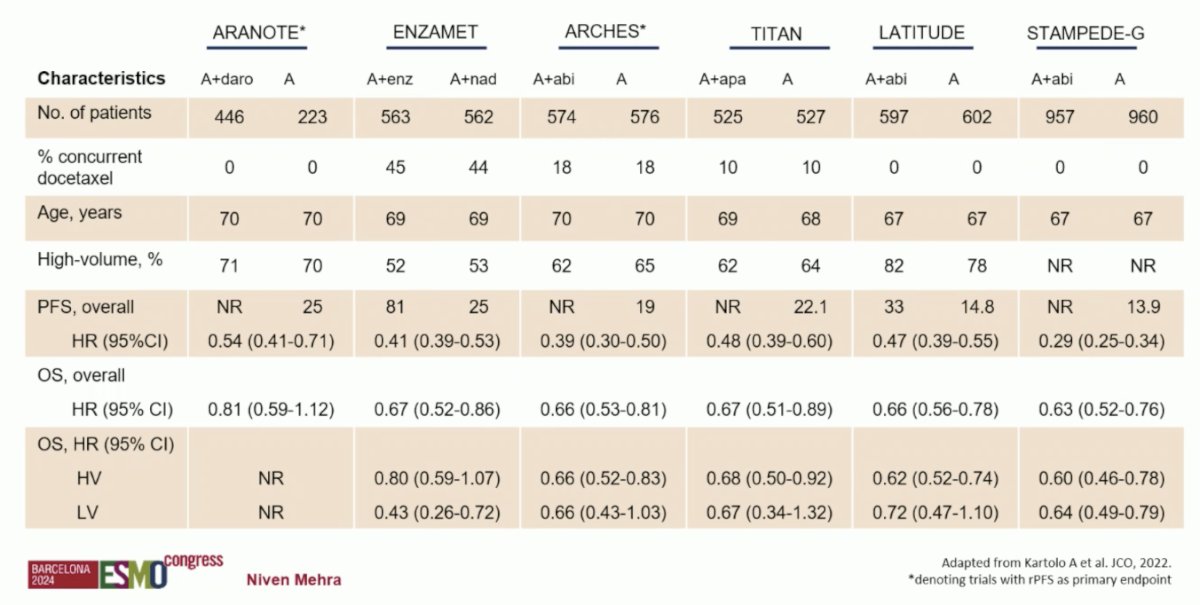
Dr. Mehra emphasized that ARANOTE was a positive study based on its primary endpoint of progression free survival, with a reduction in radiological progression or death of 46% in the overall population. So far, this has been consistent among subgroups, but there are too few events in the low volume subgroups. Importantly, this includes a population of ~30% Asian patients and ~10% Black patients. Additionally, he notes there was a very low incidence of treatment emergent adverse events between arms, and similar proportions in the experimental and control arm for grade 3-4 adverse events, grade 5 adverse events, and treatment emergent adverse events leading to permanent discontinuation. The following table highlights the context of ARANOTE to other first line mHSPC data, specifically noting that only ARCHES and ARANOTE had radiographic progression free survival as the primary endpoint:
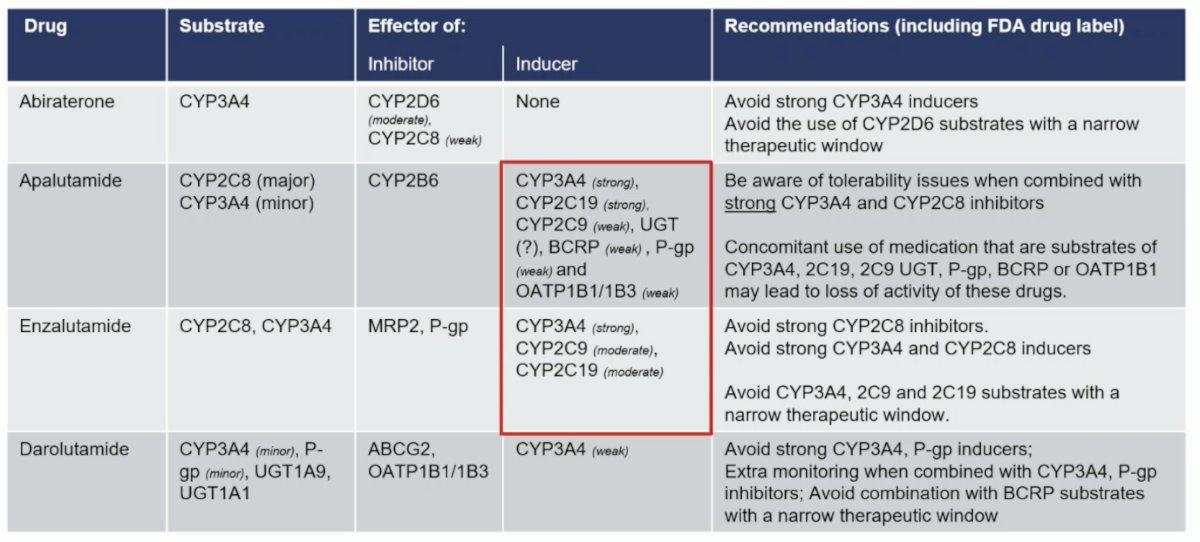
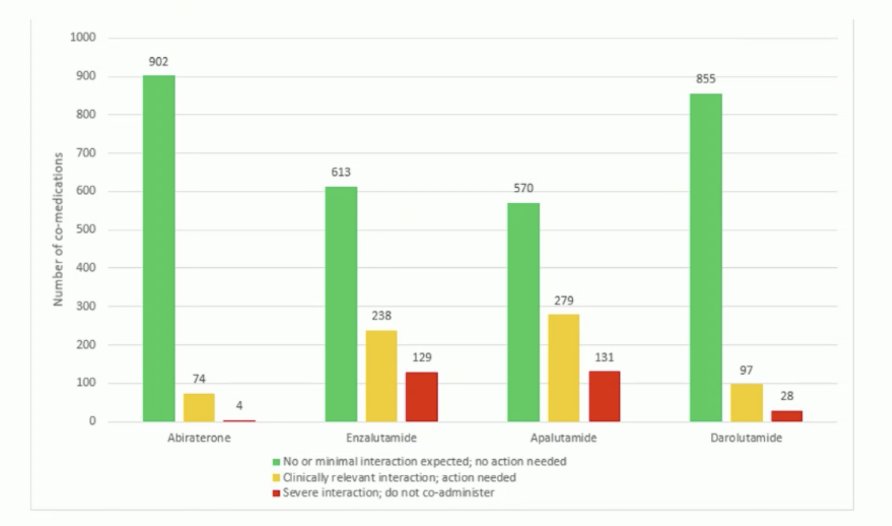
Who should receive darolutamide? Dr. Mehra states that particularly in the European Union, it will be difficult to treat all patients given that darolutamide + ADT is 4th in the market. Additionally, the EMA will be looking at cheaper options, including abiraterone which went off patent in 2023, and enzalutamide scheduled to be off patent in 2028 and apalutamide in 2031. Rather, specific patient subgroups may benefit from darolutamide based on its unique and distinct structural pharmacological properties. Generally, this will likely be the older frailer patients. Another important consideration is the potential for drug-drug interactions amongst androgen receptor pathway inhibitors. The following table notes potential interactions, specifically more common among patients receiving apalutamide or enzalutamide:
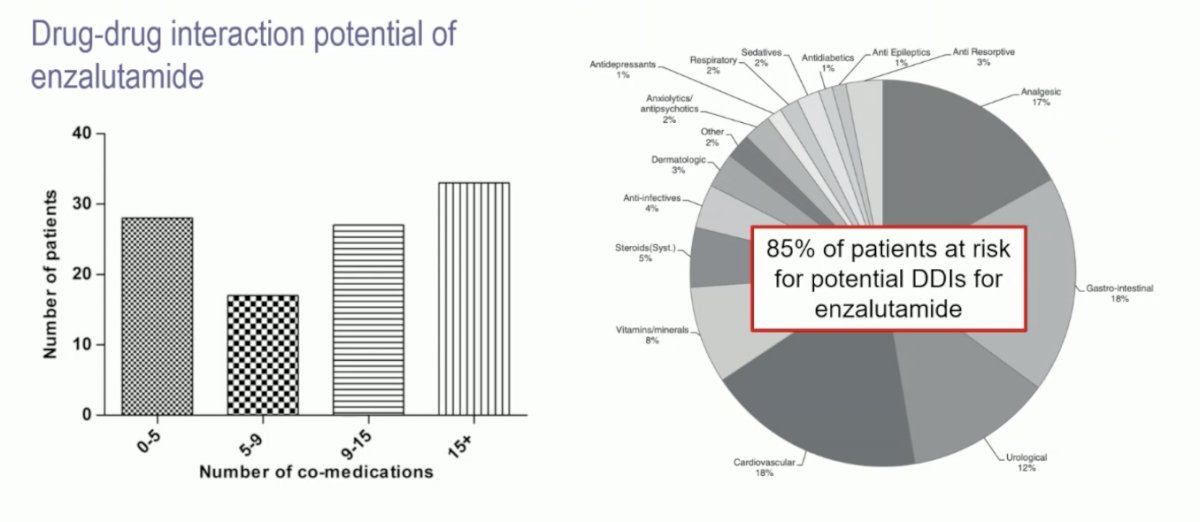
Indeed, there is a relevance for polypharmacy in elderly men with prostate cancer. A previous study from Benoist et al.1 looked at the drug-drug interaction potential in men treated with enzalutamide. They noted that many men are on >9 co-medications and that 85% of patients are at risk of a drug-drug interaction with enzalutamide:
In unpublished data from www.DDIManager.co.uk, Dr. Mehra notes that both abiraterone and darolutamide have the fewest drug-drug interactions of clinical significance:
Dr. Mehra emphasized that he thinks darolutamide will likely be best used in highly vulnerable patients. However, ARANOTE did not select for (i) patients with drug-drug interactions with abiraterone, enzalutamide, or apalutamide, (ii) elderly patients, (iii) patients with comorbidities, or (iv) ECOG 2 patients. To address this, PEACE-6 is a double-blind, randomized phase III trial evaluating the efficacy of ADT +/- darolutamide in de novo metastatic prostate cancer patients with vulnerable functional ability and not elected for docetaxel or androgen receptor targeted agents:
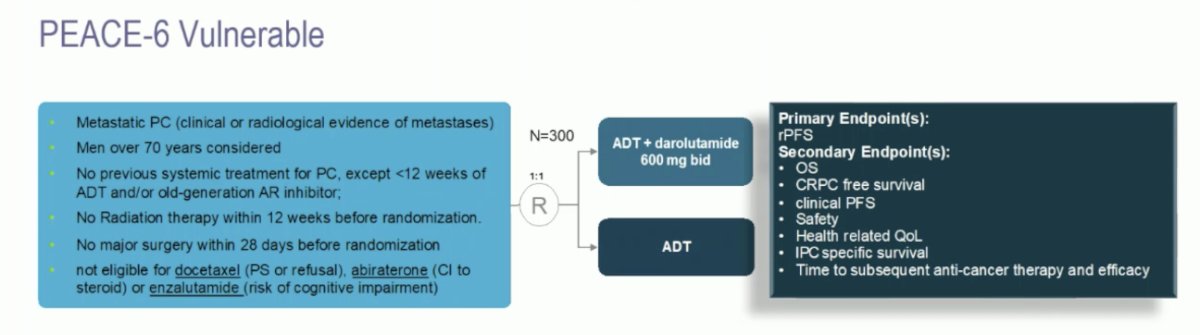
This study is open at 93 sites in 11 European countries, with 93 of 300 patients accrued to date. Dr. Mehra provided the following concluding statements with regards to ARANOTE:
- This is a phase 3 registrational trial positive for radiographic progression free survival (primary endpoint) for the FDA/EMA
- Darolutamide will face reimbursement problems in several European Union countries
- Stronger data is needed for efficacy in ‘unfit’ subpopulations, which were not including in the ARANOTE trial design but will be addressed in PEACE-6
- Individual patient data meta-analysis on efficacy, toxicity, and quality of life will be interesting
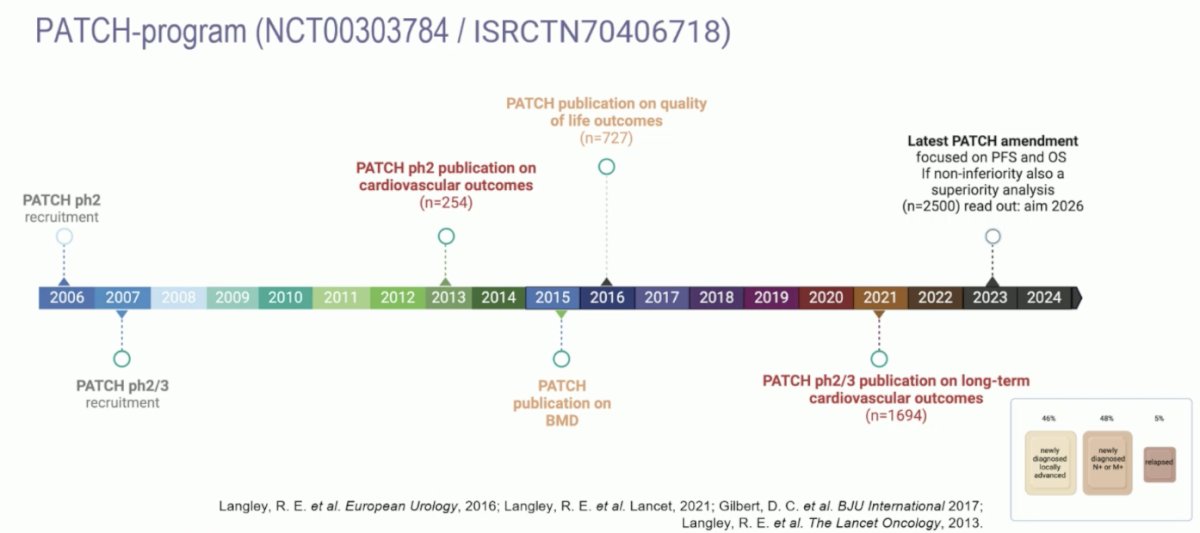
Dr. Mehra then discussed the phase 3 trial assessing transdermal estradiol versus LHRH agonist in locally advanced prostate cancer. The clinical rationale for transdermal estrogens is that it circumvents first pass hepatic metabolism and avoids cardiovascular toxicity seen with oral estrogens. Secondly, by reducing LHRH related morbidity, an improvement in overall survival may be seen with transdermal estradiol. The following figure highlights the timeline of the PATCH program assessing transdermal estrogens versus LHRH agonists:
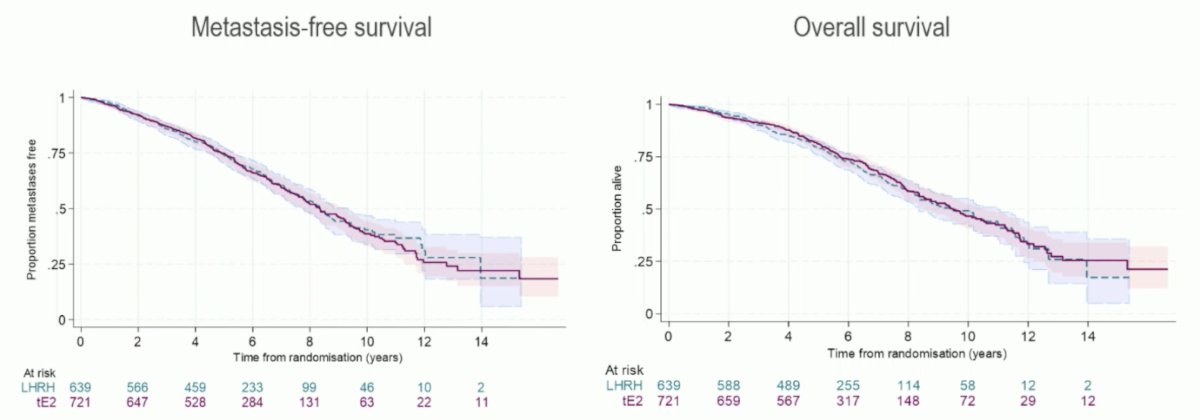
Eligibility criteria for this analysis included histologically confirmed newly diagnosed high-risk M0 (locally advanced or node positive) prostate cancer or those relapsing with PSA ≥ 4 ng/ml and doubling in <6 months, PSA ≥ 20 ng/ml, or N positive. Treatment included standard LHRH agonist versus transdermal estradiol 100 mcg/24 hour patches (four patches) changed twice weekly for ≥2 years (when testosterone <= 1.7 nmol/L, 3 patches were used and changed twice weekly); prostate radiotherapy and docetaxel were permitted. The primary outcome was metastasis-free survival, designed to rule out a >4% reduction in 3-year metastasis-free survival (85% power, 1-sided 5% α). Secondary outcomes included overall survival, castration rates, and toxicity. The PATCH study in localized prostate cancer showed non-inferiority of transdermal estradiol in metastasis free survival (primary endpoint) and no significant difference in overall survival (secondary endpoint):
Castration rates were similar but differences in adverse events were related to mechanism of action: LHRH agonist versus transdermal estradiol any grade adverse events included gynecomastia 42% versus 85% and hot flushes 89% versus 44%, respectively. Dr. Mehra emphasized that what is not reported today are differences between transdermal estradiol and LHRH agonist for toxicity (SRE, onset of osteoporosis, cardiovascular, metabolic syndrome, cognitive effects, and type 2 diabetes mellitus), quality of life, patient preference, incidence of secondary cancers, and differences between N0/N+ or local vs relapsed disease. Dr. Mehra provided the following concluding statements with regards to this PATCH analysis in localized prostate cancer:
- Transdermal estradiol is an alternative to LHRH agonist without showing any detriment with regard to metastasis free survival
- Shared decision making with our patients is necessary as there is a different toxicity profile
- Transdermal estradiol may be a suitable alternative for younger patients with normal baseline testosterone, and with cardiovascular morbidity
- Transdermal estradiol does not appear to sufficiently reduce treatment-associated morbidity to impact overall survival
- Reimbursement may be an issue, as it is currently off label to use for prostate cancer
Finally, Dr. Mehra discussed the ancillary biomarker study of the PEACE-1 phase 3 trial. There are guidelines on predictive genomic biomarkers in mCRPC, but biomarkers in CRPC are not yet proven in the mHSPC setting:
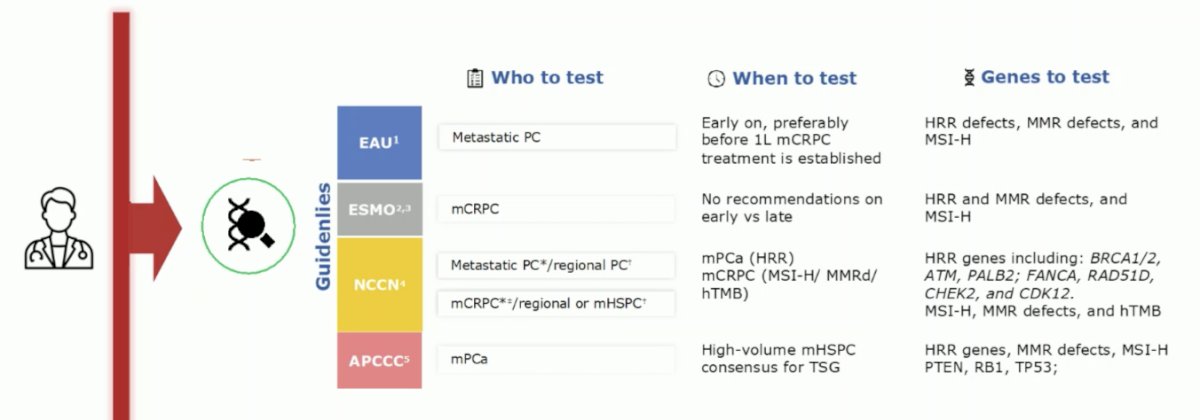
However, there are some promising biomarkers in the mHSPC disease space:
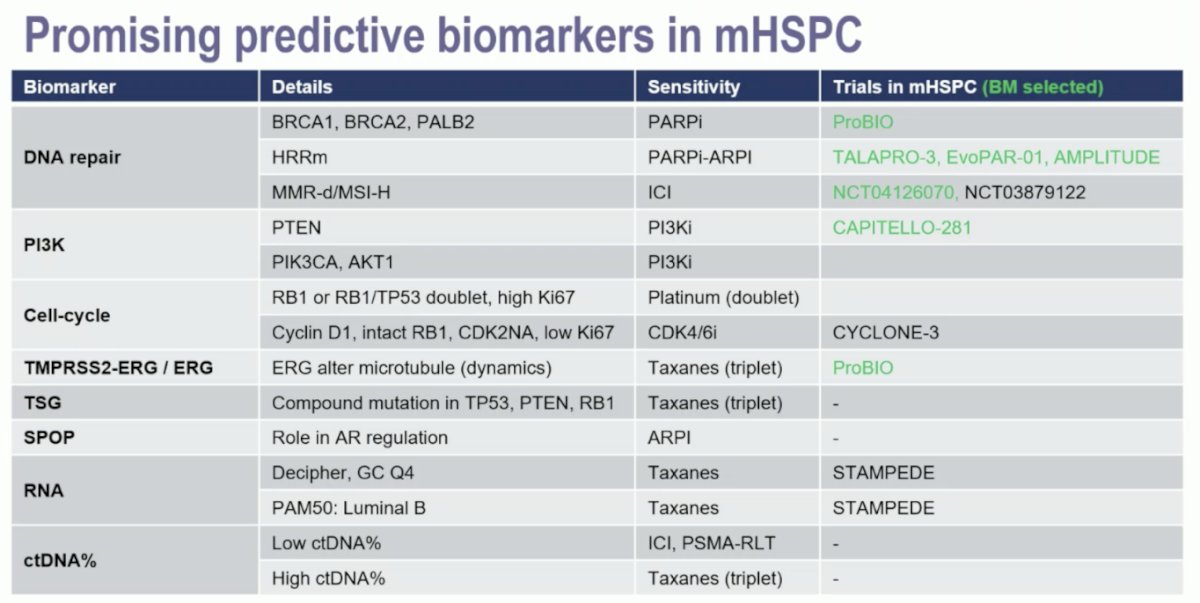
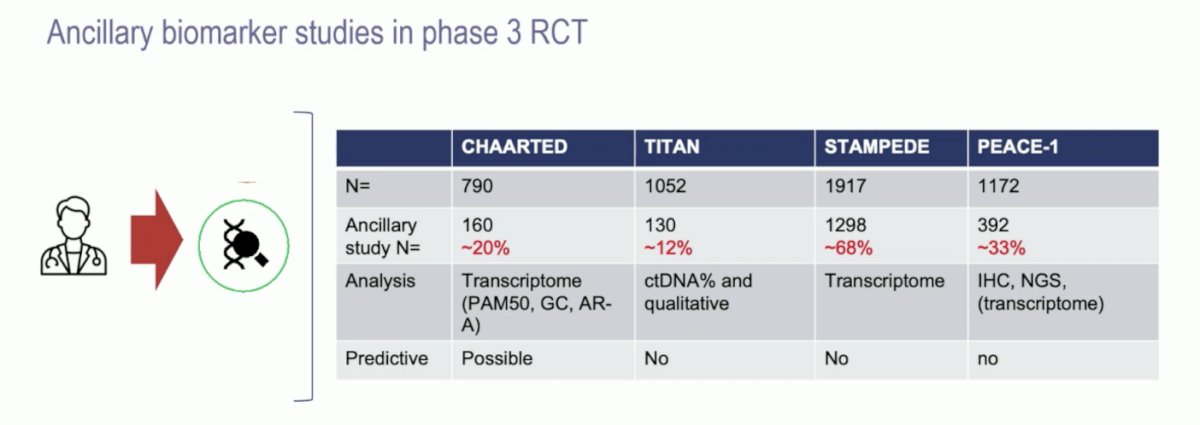
Over time, mHSPC clinical trials have done a better job of collecting and performing ancillary biomarker studies, with 33% of PEACE-1 patients, but up to 68% of patients in STAMPEDE. However, well coordinated efforts are still lacking:
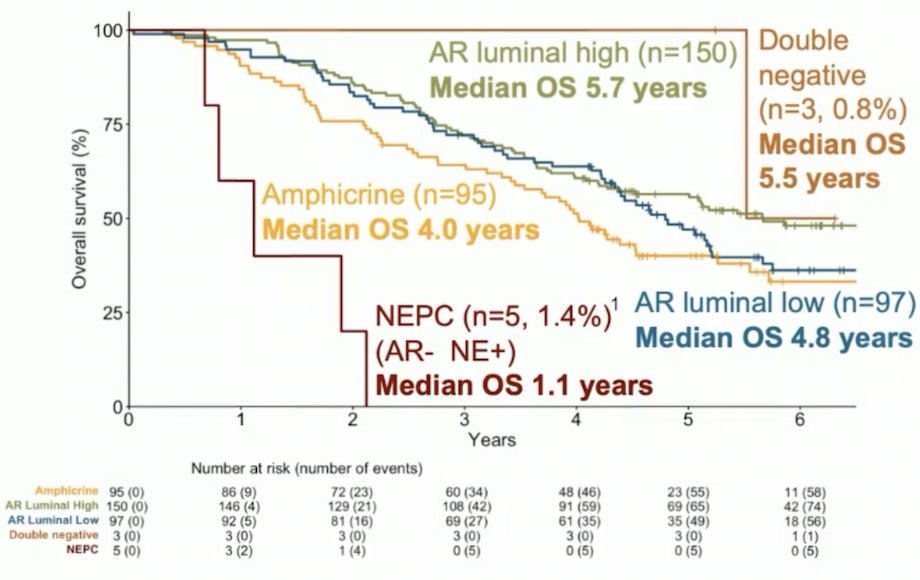
In PEACE-1, immunochemistry analyses were performed using validated antibodies staining luminal components (AR, NKX3.1), neuroendocrine features (synaptophysin, CD56, chromogranin A), tumor suppressors (p53, Rb, pTEN), Ki67, and ERG. Five phenotype subgroups were defined using luminal and neuroendocrine status. A pre-specified statistical analysis plan was approved by the steering committee, and Cox models were fitted with each immunochemistry marker adjusted for age, tumor burden, Gleason score, ECOG status, and treatments received (radiotherapy, docetaxel and abiraterone acetate + prednisone) to evaluate their prognostic value. Interaction tests between abiraterone acetate + prednisone and biomarkers were performed to assess whether these factors were predictive of treatment benefit. Among the 350 patients with an assessable phenotype, 150 (42.9%) were AR-high luminal, 97 (27.7%) AR luminal weak, 95 (27.1%) amphicrine, 3 (0.8%) double negative, and 5 (1.4%) neuroendocrine prostate cancer. No significant difference for radiographic progression-free survival or overall survival was found between these 5 subgroups but a trend from AR-high luminal (better prognosis) to neuroendocrine prostate cancer (worse prognosis), mainly driven by the neuroendocrine status:
Dr. Mehra provided the following concluding statements with regards to the PEACE-1 ancillary biomarker study:
- Data from phase 3 studies assessing predictive/companion biomarkers are still lacking in mHSPC, thus data from well-designed ancillary studies are also limited
- No predictive biomarkers were identified for abiraterone
- Standardization is necessary for biomarkers (assessment), and collection/pre-analytical processing to allow for extrapolation of results between trials (ie. transcriptome)
Presented by: Niven Mehra, MD, Radboud University Medical Center, Nijmegen, Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
Related Content:ESMO 2024: Efficacy and Safety of Darolutamide plus ADT in Patients with mHSPC from the Phase 3 ARANOTE Trial
ESMO 2024: Prostate Cancer Efficacy Results from a Randomized Phase 3 Evaluation of Transdermal Estradiol Versus LHRH Agonists for Androgen Suppression in M0 Prostate Cancer


