(UroToday.com) The 2024 ESMO annual meeting included a session on prostate cancer, featuring a discussant presentation by Dr. Christopher Sweeney discussing two abstracts including “CC-94676-PCA-001: Clinical activity of BMS-986365, a dual androgen receptor ligand-directed degrader and antagonist, in heavily pretreated patients with mCRPC” by Dr. Dana Rathkopf and “INSPIRE: Phase 2 trial of Nivolumab 3mg/kg and ipilimumab 1mg/kg in molecularly selected patients with mCRPC” by Dr. Niven Mehra. Dr. Sweeney started his discussant presentation by highlighting key features of metastatic CRPC treatment in 2024 and beyond:
- Prior ADT + androgen receptor pathway inhibitor
- The androgen receptor pathway inhibitors include: abiraterone, apalutamide, enzalutamide or darolutamide
- +/- docetaxel, +/- radiation to the primary
- The patients will be frailer and older after years of ADT + androgen receptor pathway inhibitors
- Patients will have disease that is less dependent on the androgen receptor
- There will be more available therapies and biomarkers
- It is imperative to ensure patients get optimal first-line mCRPC treatment:
- We need to personalize treatment to match cancer biology, frailty, health, and the patient’s social situation
- Optimize the maximum change of benefit (less resistance)
- There are limited numbers of patients that get to second-line mCRPC treatment in the real world
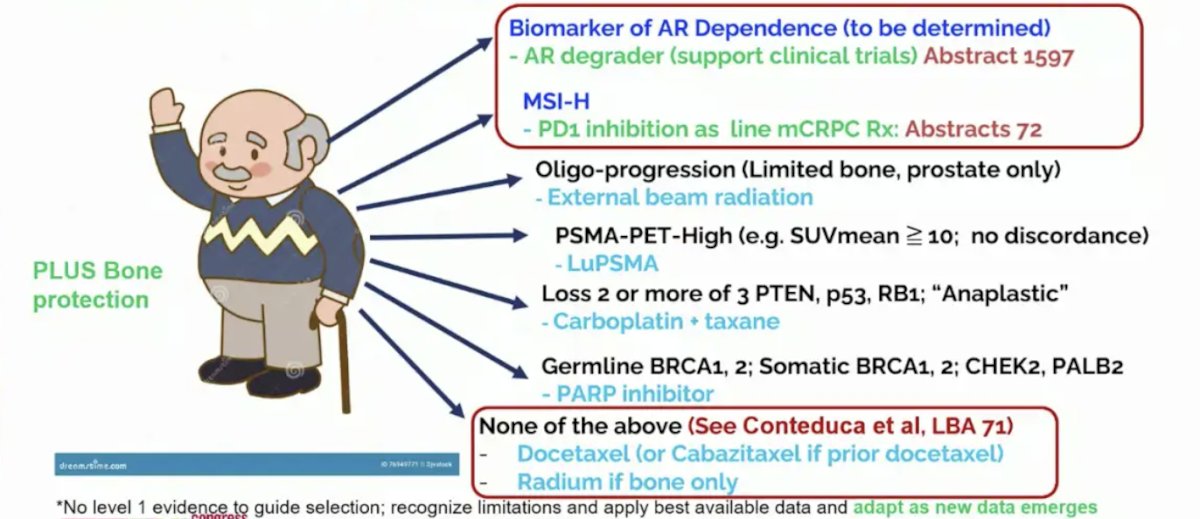
For a patient with newly diagnosed mCRPC, there are several personalized options for mCRPC after ADT + androgen receptor pathway inhibitors depending on the clinical scenario:
- Oligo-progression (limited bone, prostate only): external beam radiation
- PSMA PET high (ie. SUVmean >= 10, no discordance): LuPSMA
- Loss of 2 or more of 3 PTEN, p53, RB1 (“anaplastic”): carboplatin + taxane
- Germline BRCA1/2, somatic BRCA1/2, CHEK2, PALB2: PARP inhibitor
- None of the above: docetaxel (or cabazitaxel if prior docetaxel) or Radium-223 if bone only
- From ESMO 2024 – Biomarker of AR dependence (to be determined): AR degrader (a possible new agent)
- From ESMO 2024 – MSI-H: PD1 inhibition
Dr. Sweeney notes that good personalized mCRPC treatment is not good enough until we can cure the disease. In VISION,1 for patients with an SUVmean >10, median overall survival was 21 months, and only 14 months for SUVmean < 10. With regards to PARP inhibitors, PARP inhibition activity depends on “dependence” on the given DDR pathway. A DDR mutation does not equate to a PARP inhibitor benefit:
Dr. Sweeney emphasized that an AR degrader may work in mCRPC given that there is a spectrum of AR dependency in metastatic prostate cancer. When tumors are very AR dependent the androgen receptor is the major driver, when modestly AR dependent the androgen receptor is present and a partial driver, and when tumors are not AR dependent the androgen receptor is present or absent and there are multiple other drivers:
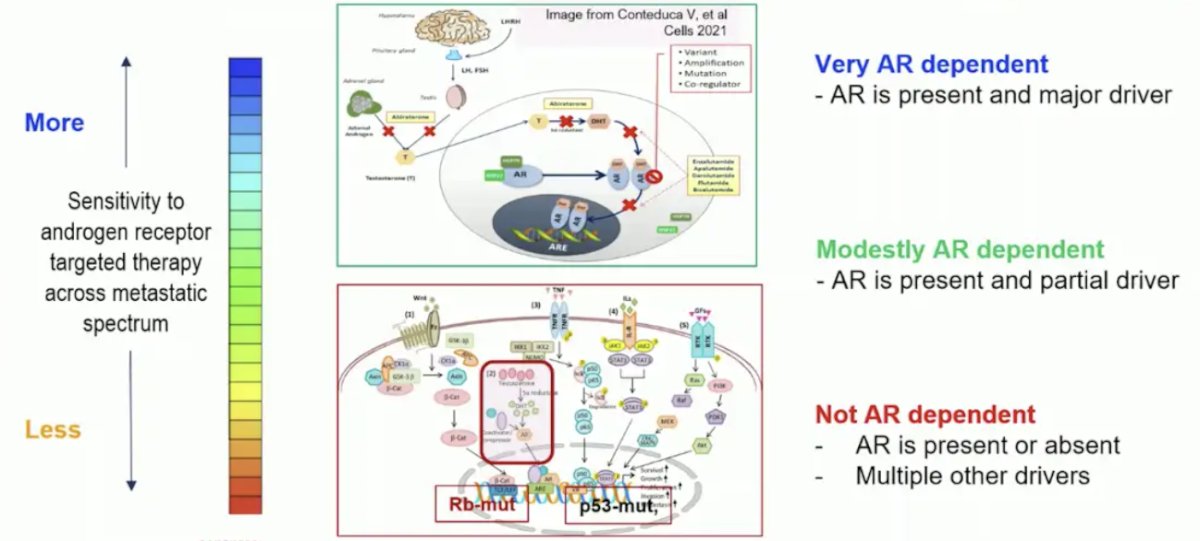
However, we do not know the percentage of patients with mCRPC with AR dependent disease after prior ADT + androgen receptor pathway inhibitors, thus we need predictive biomarkers. Dr. Sweeney notes that there is variable AR dependence in mHSPC, with more in metachronous low volume disease, as seen in ENZAMET2 and ARANOTE:
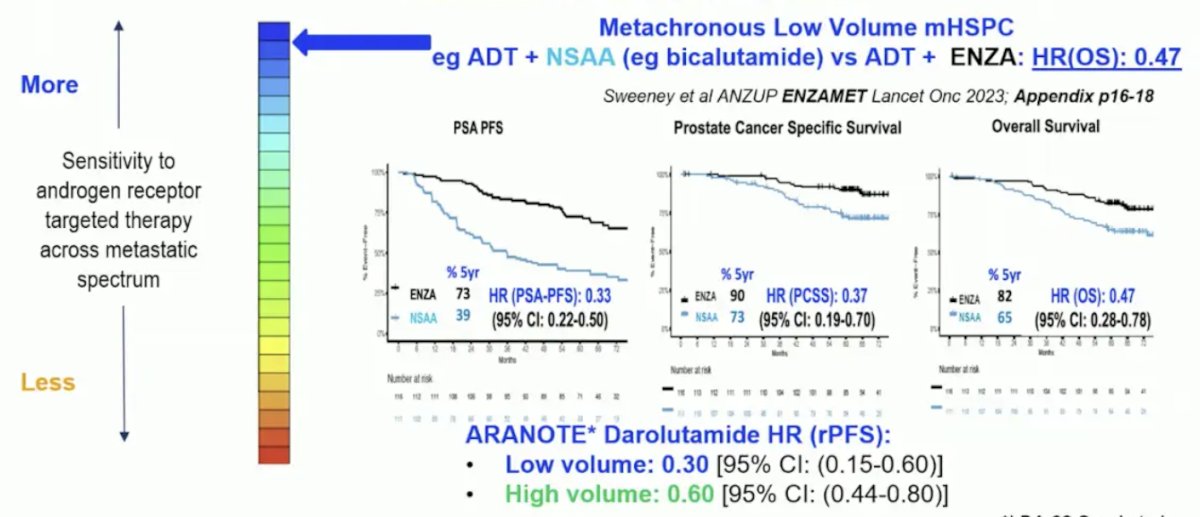
And there is less AR dependence in synchronous high volume disease, as seen in ENZAMET and PEACE-1:3
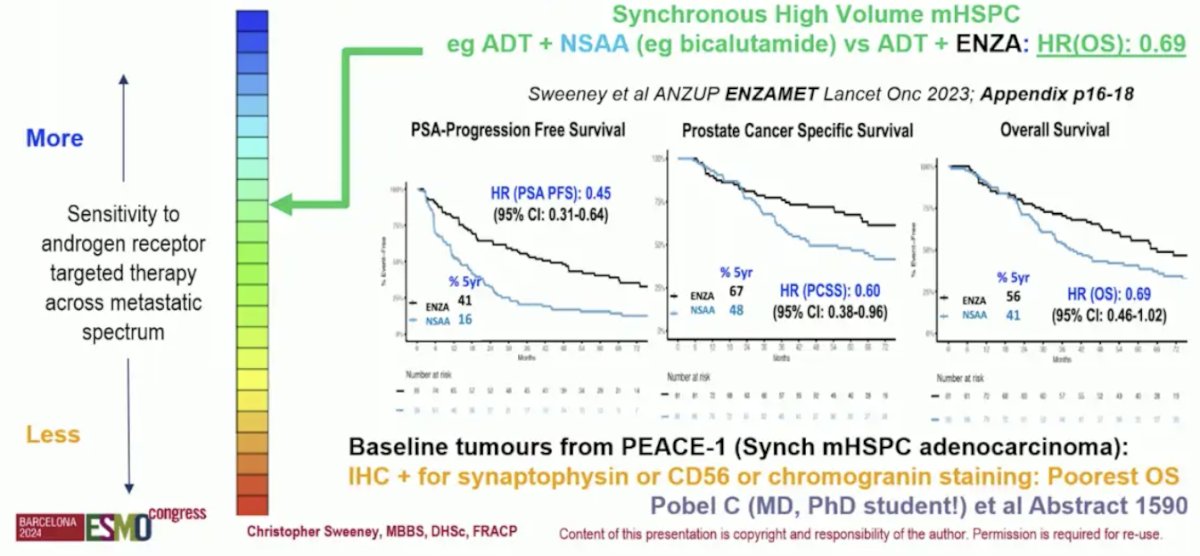
After testosterone suppression alone, the androgen receptor is upregulated as a compensatory survival pathway. There is modest dependency and rationale for modest overall survival mCRPC benefit with abiraterone and enzalutamide, but other pathways emerge. Further, there is modest AR dependency of mCRPC after ADT alone. This is noted by abiraterone or enzalutamide after ADT alone with hazard ratios for OS ranging from 0.70-0.80 and median overall survival of 3 years. However, there is variable AR dependence of metastatic prostate cancer after ADT + androgen receptor pathway inhibitors, as evidence for enzalutamide and abiraterone switch:
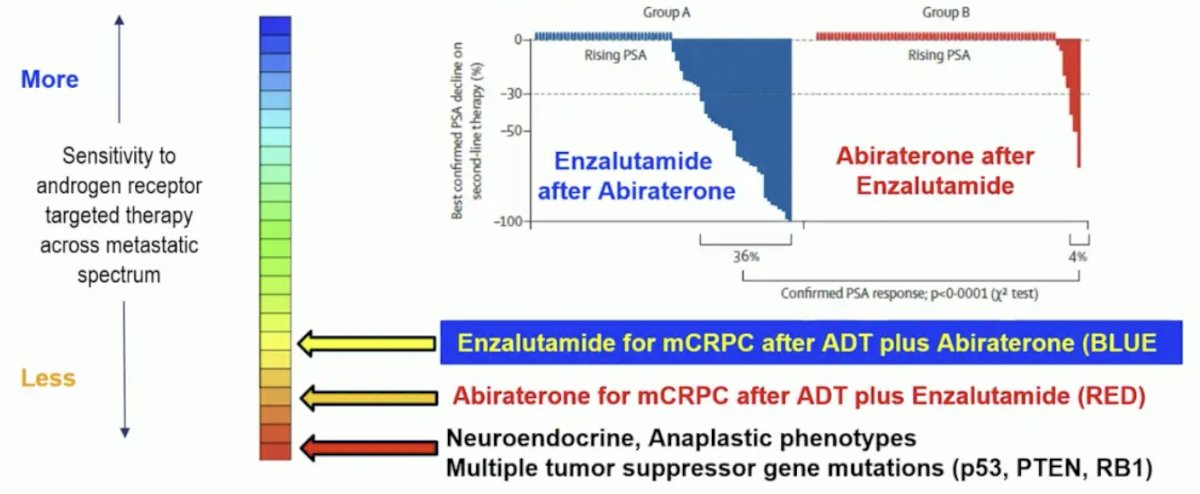
One potential biomarker of more AR dependence is ctDNA <2% (lower tumor burden) suggesting a better mCRPC prognosis:
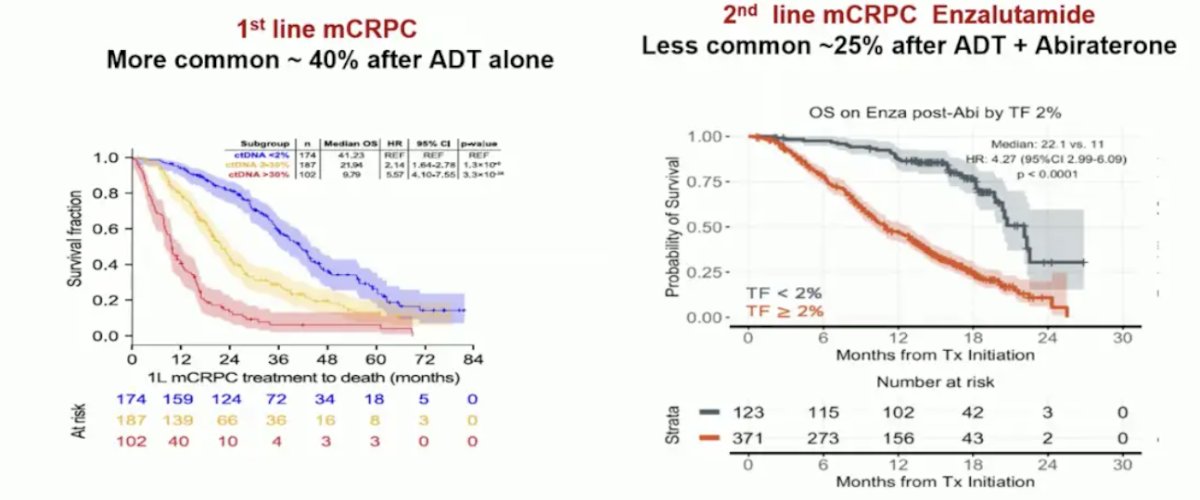
Potential biomarkers for less AR dependence include tumor suppressor gene mutations (p53, RB1, PTEN) in both tissue and ctDNA >2%, which are associated with poor mCRPC outcomes.
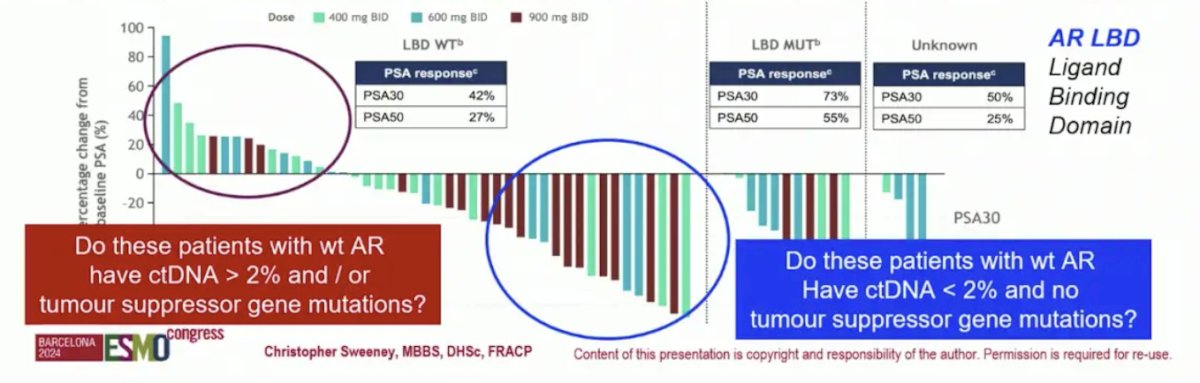
Dr. Sweeney highlighted that for the AR degrader BMS-986365, there is encouraging data. PROTAC AR degraders are still modestly AR dependent, but what are the other biological features for more personalization? What is the percentage of responders outside of phase 1 trials with less clinical selection? In the BMS-986365 trial, clinical benefit was observed both in patients with AR ligand binding domain WT and mutant mCRPC. But, do the poor responders with wt AR have ctDNA >2% and or tumor suppressor gene mutations? And do the good responders with wt AR have ctDNA <2% and no tumor suppressor gene mutations?
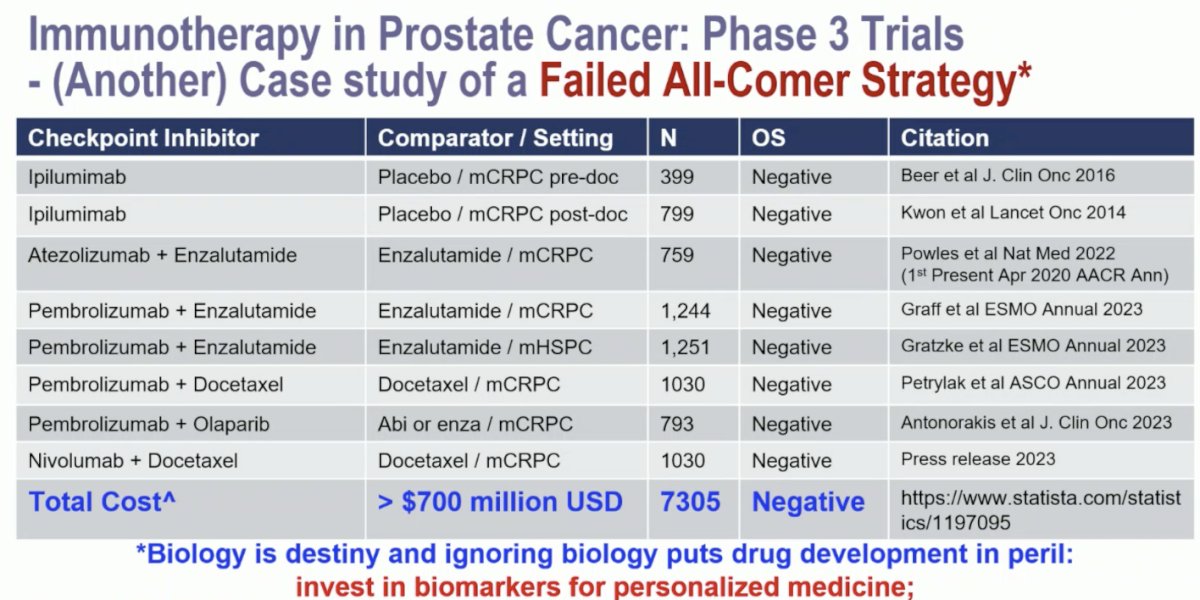
Dr. Sweeney emphasized that phase 3 trials of immunotherapy in prostate cancer are case studies of a failed all-comer strategy. To his estimation, the total cost of these trials is >$700 million USD among 7,305 patients for all 8 negative trials:
On the contrary, Dr. Mehra and colleagues’ approach to selected patients for nivolumab + ipilimumab in mCRPC is a case study of effective personalized mCRPC therapy. MMRd patients had a clinically meaningful and durable benefit, however, it took screening of 460 patients to identify 17 (3%) MMRd patients, who had a PSA50 rate of 86%. However, overall these patients had ~20% Grade 3 immune related toxicities, 10% with life changing toxicities, and 2% with treatment related death. So, is PD(L)1 alone enough (not need ipilimumab)? Certainly, toxicity goes up and efficacy goes down in the real-world setting.
Dr. Sweeney concluded his presentation by again highlighting the best personalized medicine as first line mCRPC among patients who have progressed on ADT + ARPI (ESMO24):
Presented by: Christopher Sweeney, MBBS, South Australian Immunogenomics Cancer Institute, University of Adelaide, Adelaide, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019 Jul 11;381(2):121-131.
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.