(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a session on future therapy targets for metastatic prostate cancer patients. Dr. Wilbert Zwart discussed targeting epigenetic pathways as a potential therapeutic target for metastatic prostate cancer.
Epigenetics is the study of heritable traits, or a stable change of cell function, that happens without changes to the DNA sequence. Epigenetics typically involves modification of DNA, including DNA methylation, or histone proteins. The epigenetic space is of interest for ongoing drug development, many of which have since been approved by the US Food and Drug Administration (FDA).
Prostate cancer is under tight epigenetic control. One important epigenetic target in prostate cancer is H3K27ac which is undergoes methylation/de-methylation to allow to facilitate gene suppression/expression. 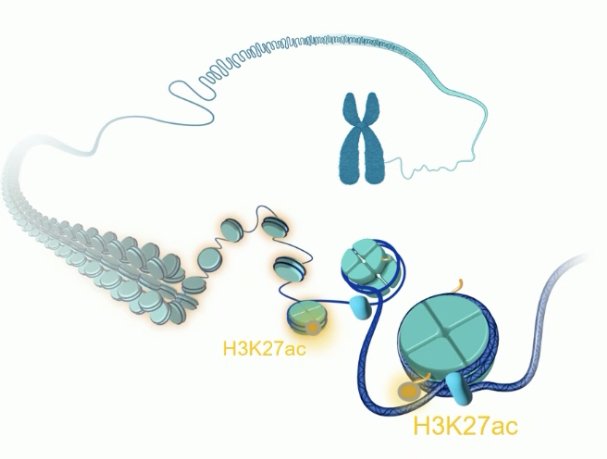
Epigenomics on clinical samples is typically performed using chromatin immunoprecipitation (ChIP)-seq analyses, whereby tissue is crosslinked, then undergoes shear chromatin, immunoprecipitation, and the DNA is finally sequenced. This technology has yielded an ∼100% success rate for all transcription factors analyzed.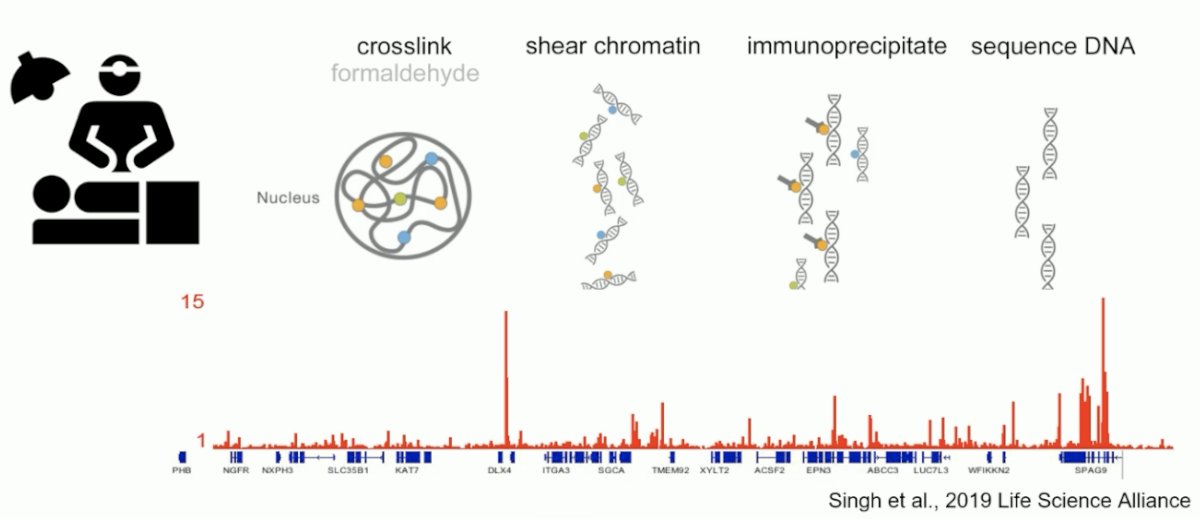
Dr. Zwart’s group was able to apply this technology to tissue obtained from an enlarged pelvic lymph node in a prostate cancer patient to determine which where androgen receptor and H3K27ac bind.
These epigenetic studies have helped elucidate the plasticity of prostate cancer cells. Androgen receptor DNA binding is heavily re-programmed in tumorigenesis, moves to new locations upon specific protein-coding mutations, and evolves during disease progression and adaptation to therapy. In autopsy programs, Dr. Zwart’s group was able to demonstrate reproducibility between different metastases from the same patient. These sites are indicative of context-dependent patient prognostication.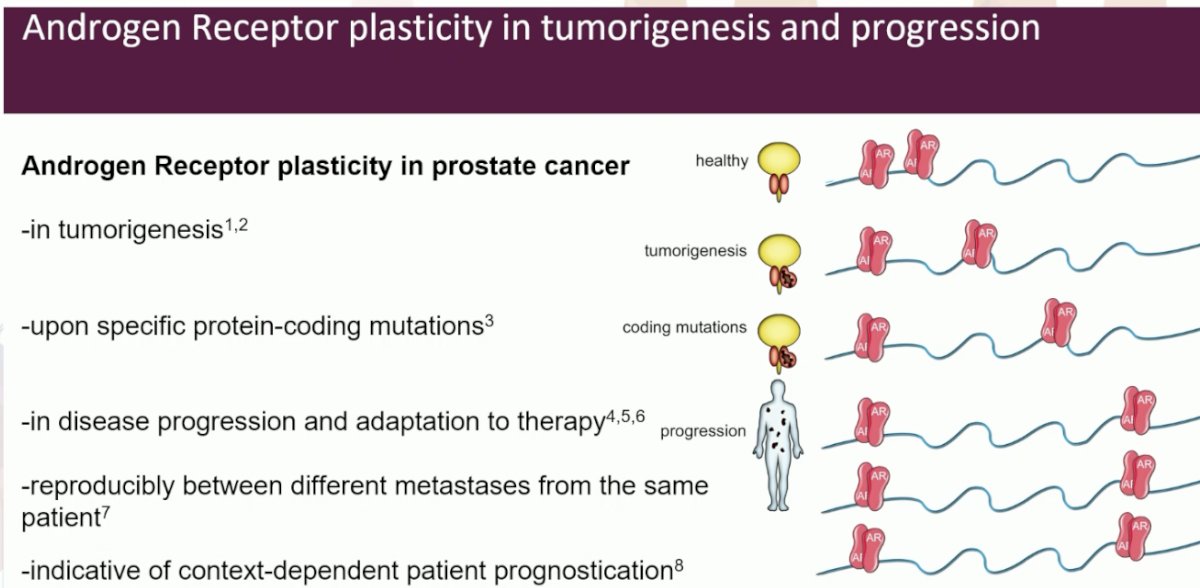
Dr. Zwart noted that these findings were based on retrospective work, and his group is now attempting to investigate this in a prospective fashion in both the mCRPC and neoadjuvant settings. Epigenetic analyses in clinical trials in search of biomarkers for response to androgen receptor-targeted therapy are underway.
In the neoadjuvant study, Dr. Zwart and colleagues focused on both the androgen receptor (AR), which is essential for prostate cancer growth, and the protein FOXA1, which is a transcription factor that opens chromatin at specific sites to enable AR to bind.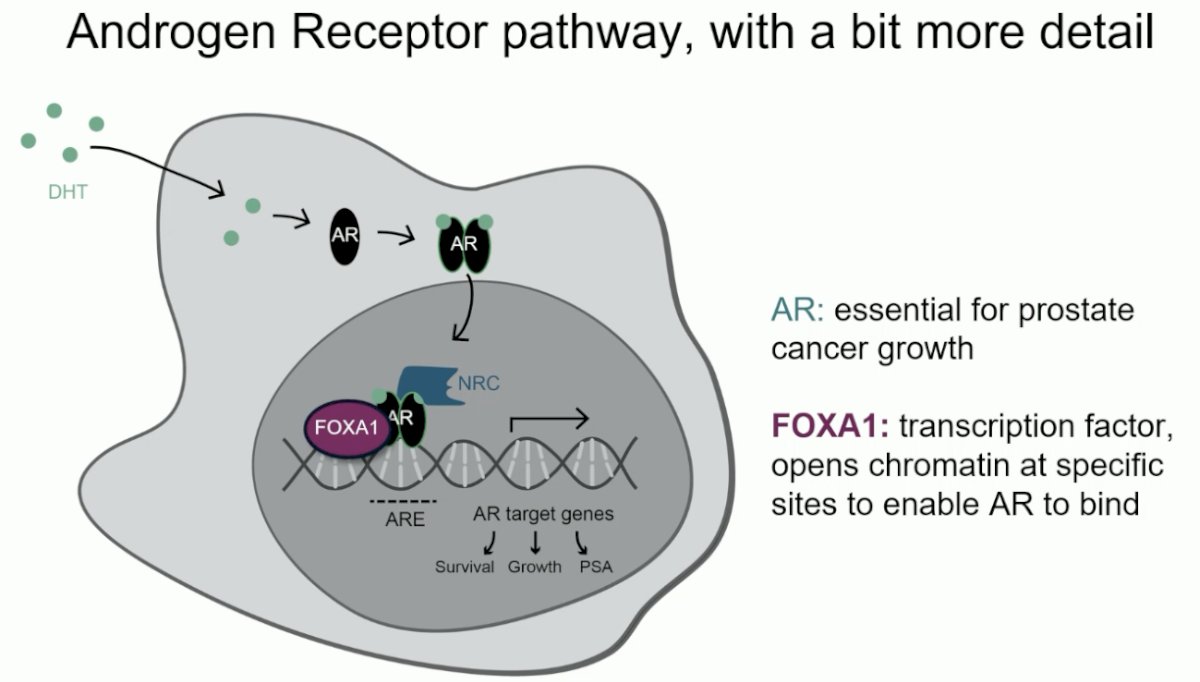
In the neoadjuvant setting, Dr. Zwart and colleagues performed integrative multiomics analyses on tissues isolated before and after 3 months of AR-targeting enzalutamide monotherapy from patients with high-risk prostate cancer enrolled in a neoadjuvant clinical trial. Transcriptomic analyses demonstrated that AR inhibition drove tumors toward a neuroendocrine-like disease state.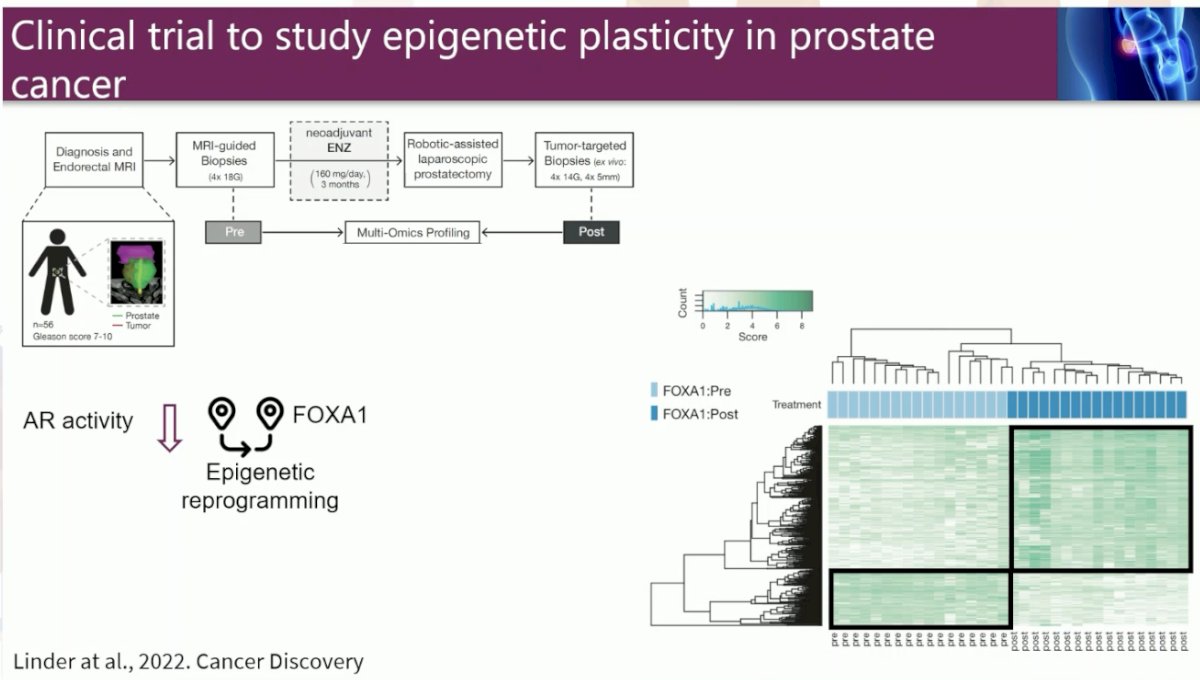
Additionally, epigenomic profiling revealed massive enzalutamide-induced reprogramming of pioneer factor FOXA1 from inactive chromatin sites toward active cis-regulatory elements that dictate prosurvival signals. Notably, treatment-induced FOXA1 sites were enriched for the circadian clock component ARNTL.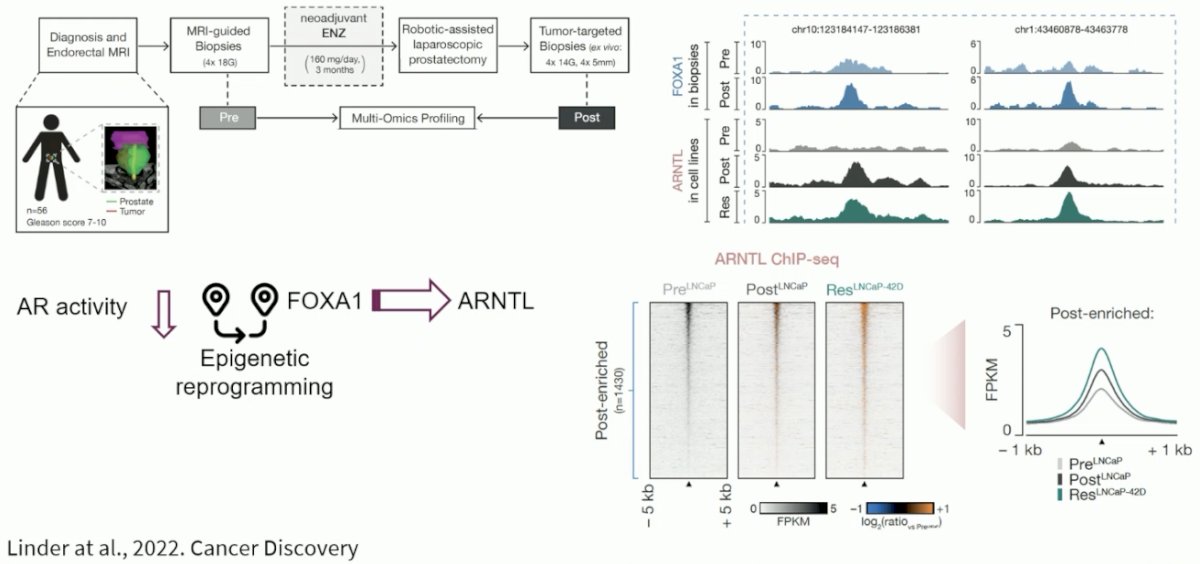
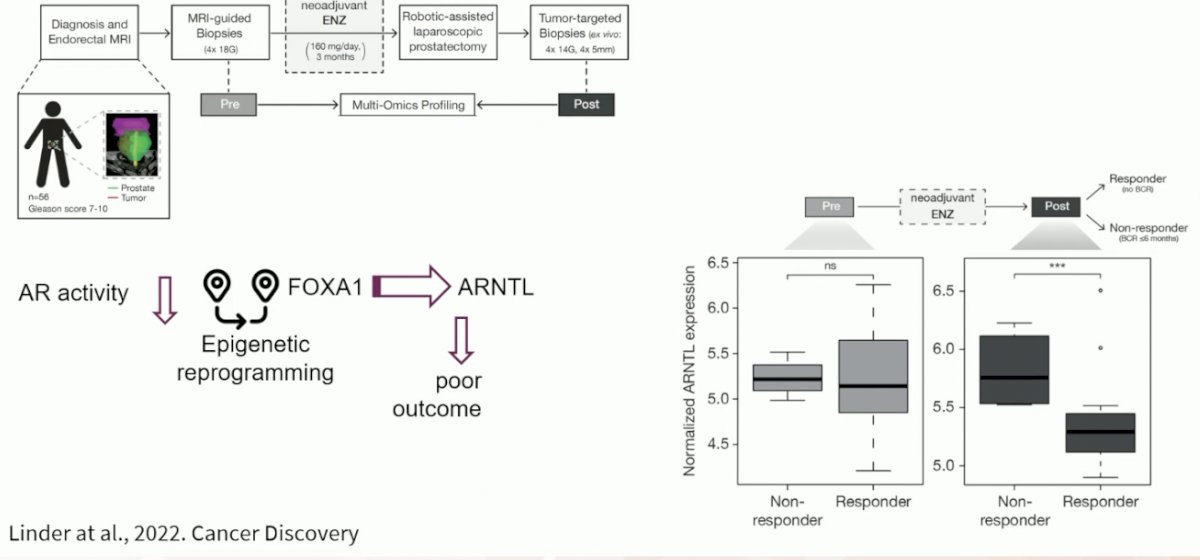
Posttreatment ARNTL levels were associated with patients' clinical outcomes and ARNTL knockout strongly decreased prostate cancer cell growth.
This data highlighted a remarkable cistromic plasticity of FOXA1 following AR-targeted therapy and revealed an acquired dependency on the circadian regulator ARNTL, a novel candidate therapeutic target.2
Dr. Zwart noted that what makes ARNTL interesting is that ‘it has nothing to do with prostate cancer’. It has classically been recognized as a circadian rhythm regulator.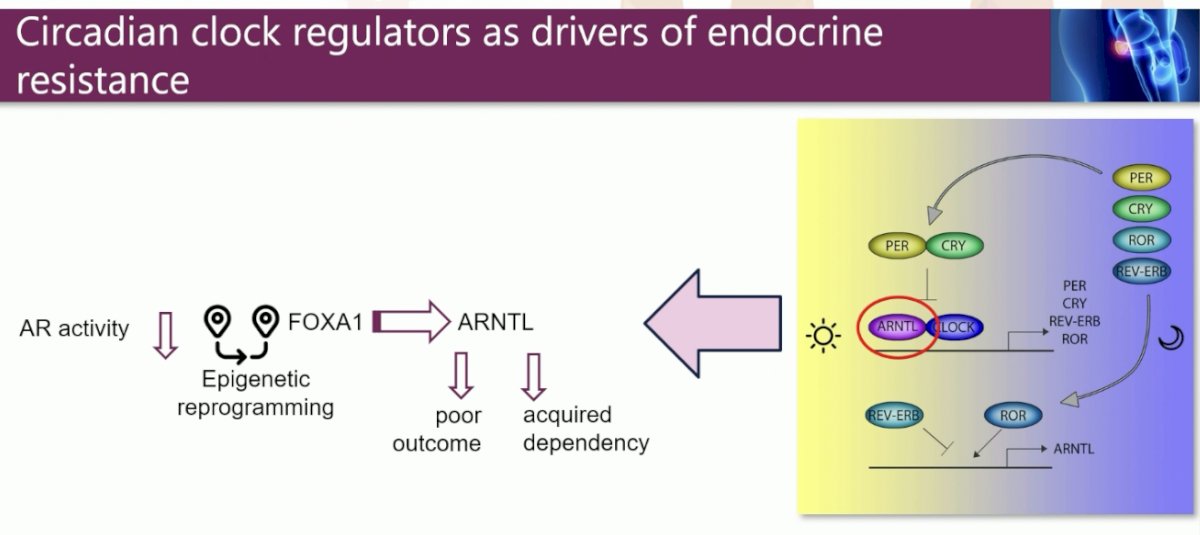
ARNTL has thus been the subject of ‘biologic repurposing’ moving from circadian regulation towards driver of endocrine resistance in cancer.
Next, moving on to discuss the ongoing data in the mCRPC space. This study included 60 mCRPC patients with PSA and/or radiographic progression who had not received prior enzalutamide. These patients underwent a prostate tissue biopsy prior to starting treatment with ADT + enzalutamide/abiraterone and then another biopsy 12 weeks post-treatment initiation. They assessed epigenetic features of lymph node, liver, and bone metastases. They selected all samples with ≥30% tumor cells.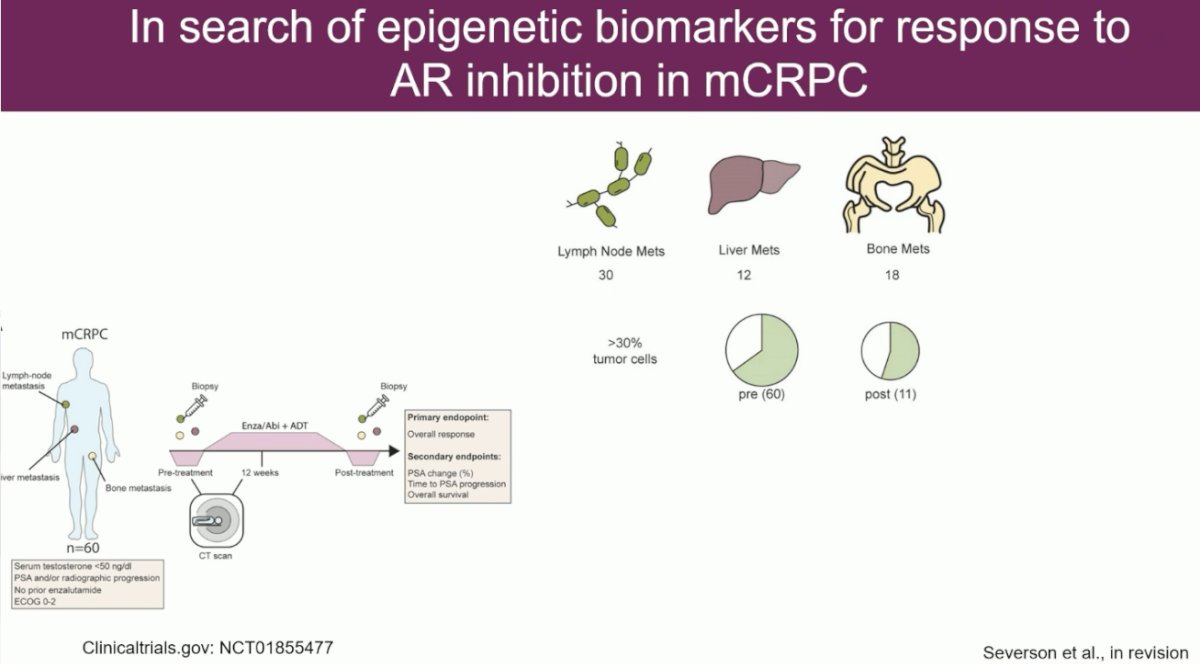
Epigenetic profiling demonstrated that the global H3K27ac profiles demarcating active promoters and enhancers were largely comparable between the response groups. Furthermore, there was no association between the principal component number and response type (responder, intermediate, non-responder).
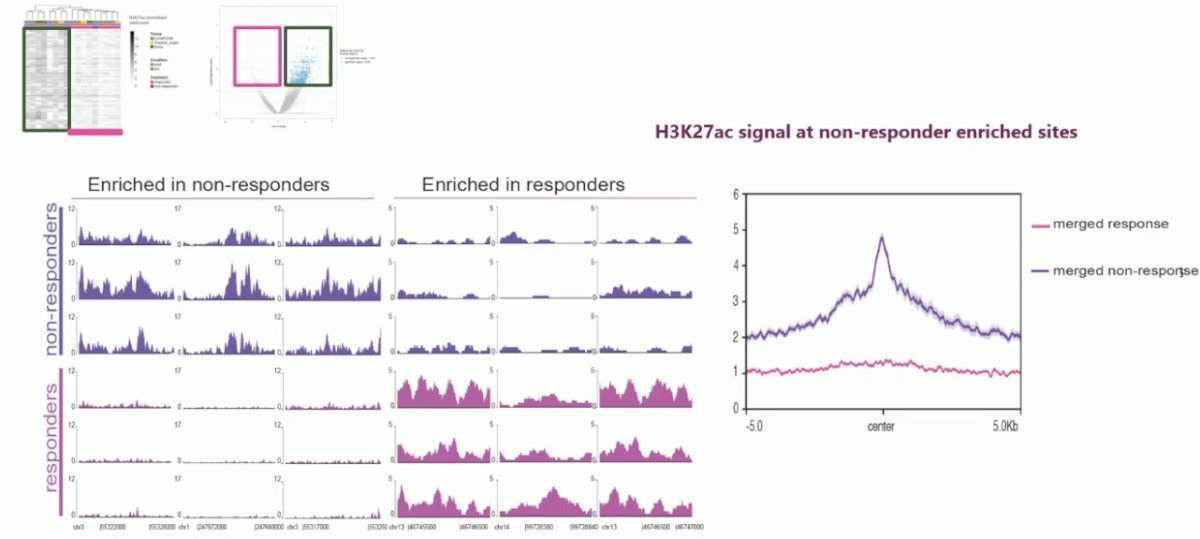
They were next able to identify regions which demarcate hallmarks of resistance or sensitivity to treatment. They performed a supervised analysis, and this identified 6,057 regions which completely and strongly demarcate whether patients are sensitive or resistant to therapy.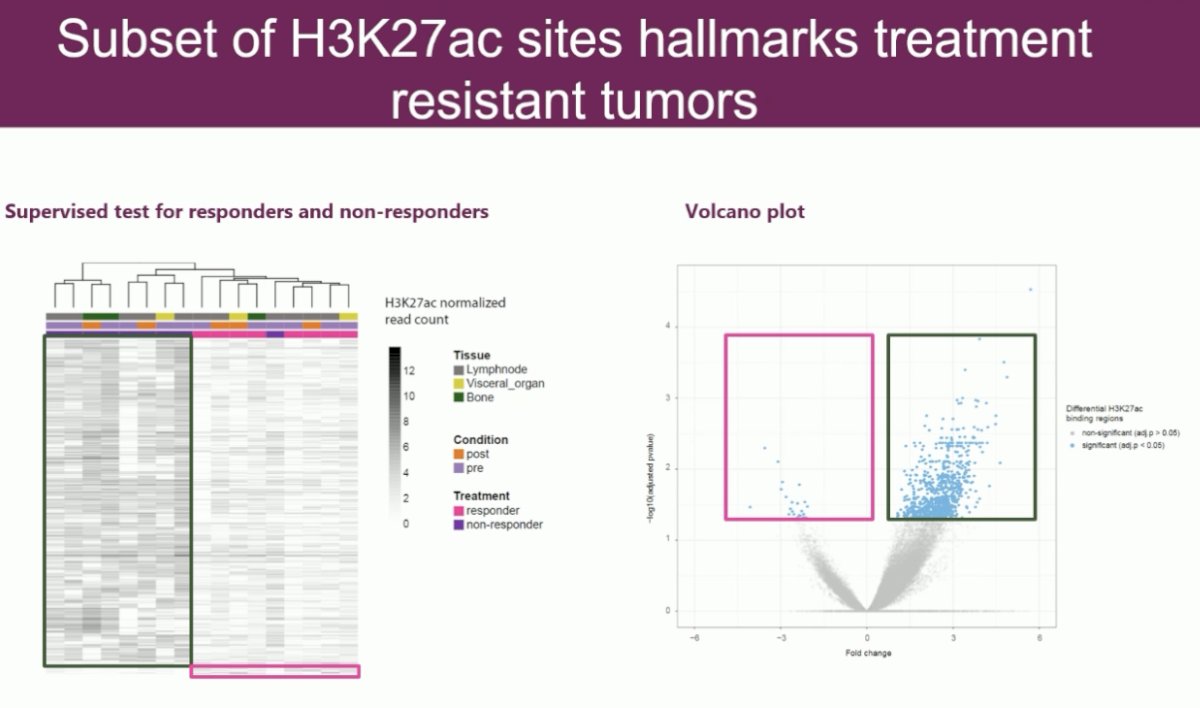
Next, a validation of these results was performed in a PDX mouse model. mCRPC tumors were isolated from patients and transferred to the mice. These mice were then either castrated or left intact.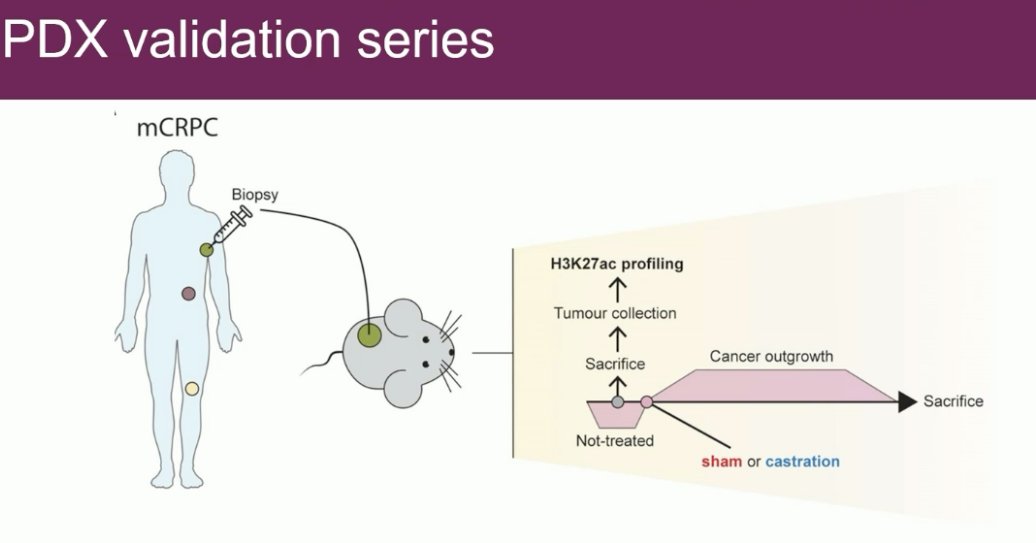
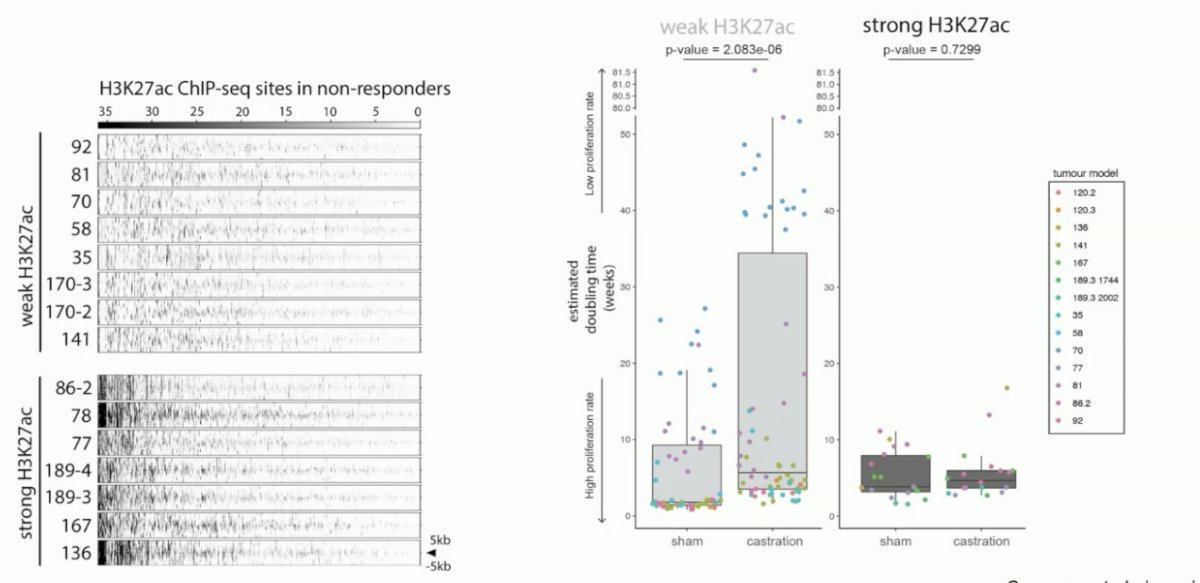
Next, they coupled the castration data with the epigenetic data. They observed differential H3K27ac profiles in the mCRPC PDX models, where there was either a strong or weak H3K27ac signal in the H3K27ac ChIP-seq sites.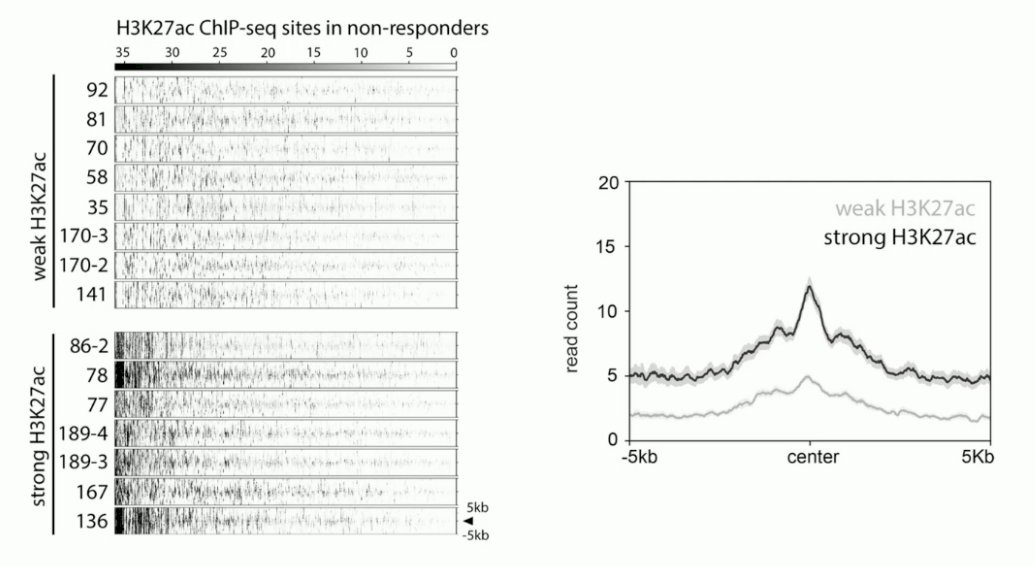
In tumors with weak H3K27ac signaling, there was an excellent response to castration. Conversely, in tumors with strong H3K27ac signaling for the resistance marker, there was no response to castration.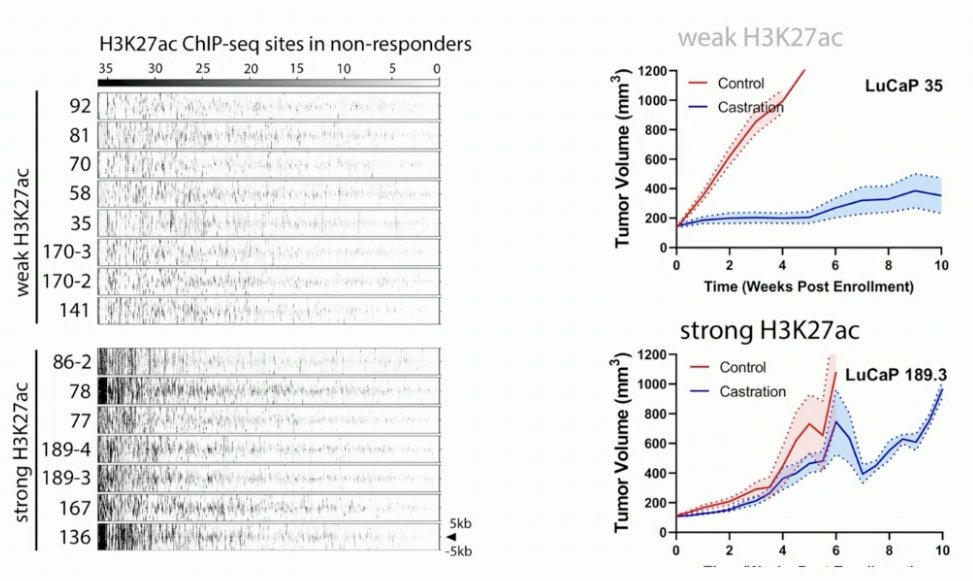
Which proteins drive treatment resistance? In an analysis of 40,000 chromatin-binding proteins, they identified influential proteins, including TOP1, NR3C1, and FOXA1. They next performed a functional proteination experiment performing knockdowns of each of these proteins in castrate-resistant cells to assess whether this would have an impact. As seen in red below, knockdown of each these proteins had an impact on cellular proliferation.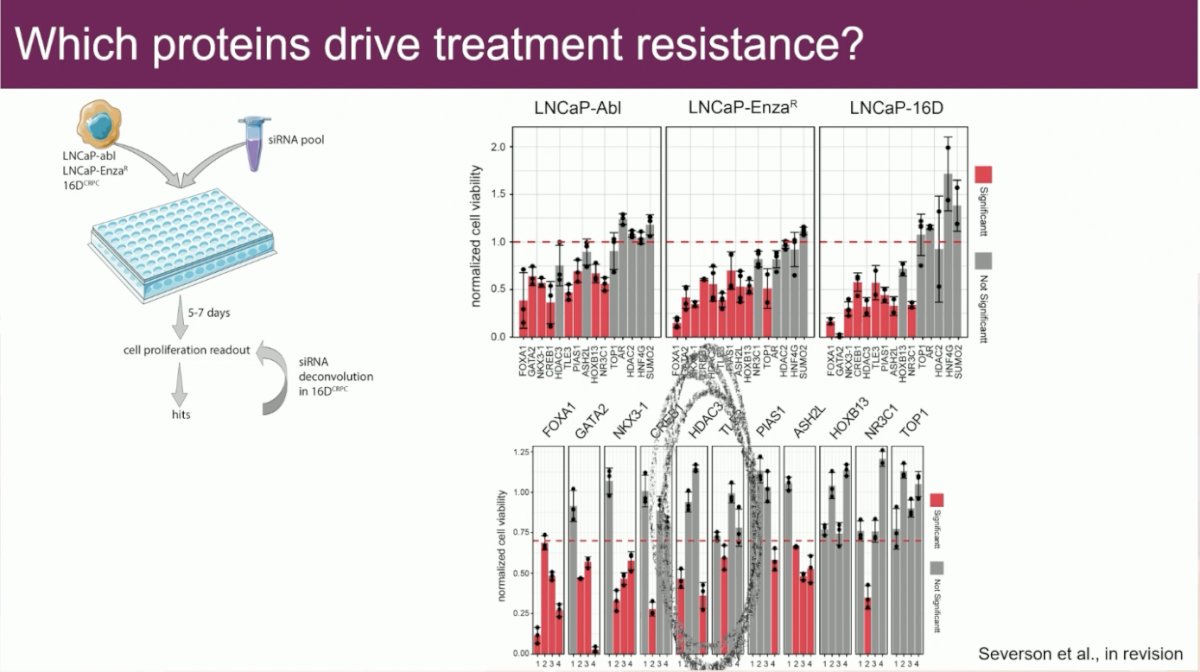
One of these targetable proteins is HDAC3. Promisingly, there appears to be drug synergy between enzalutamide and the HDAC inhibitor, vorinostat.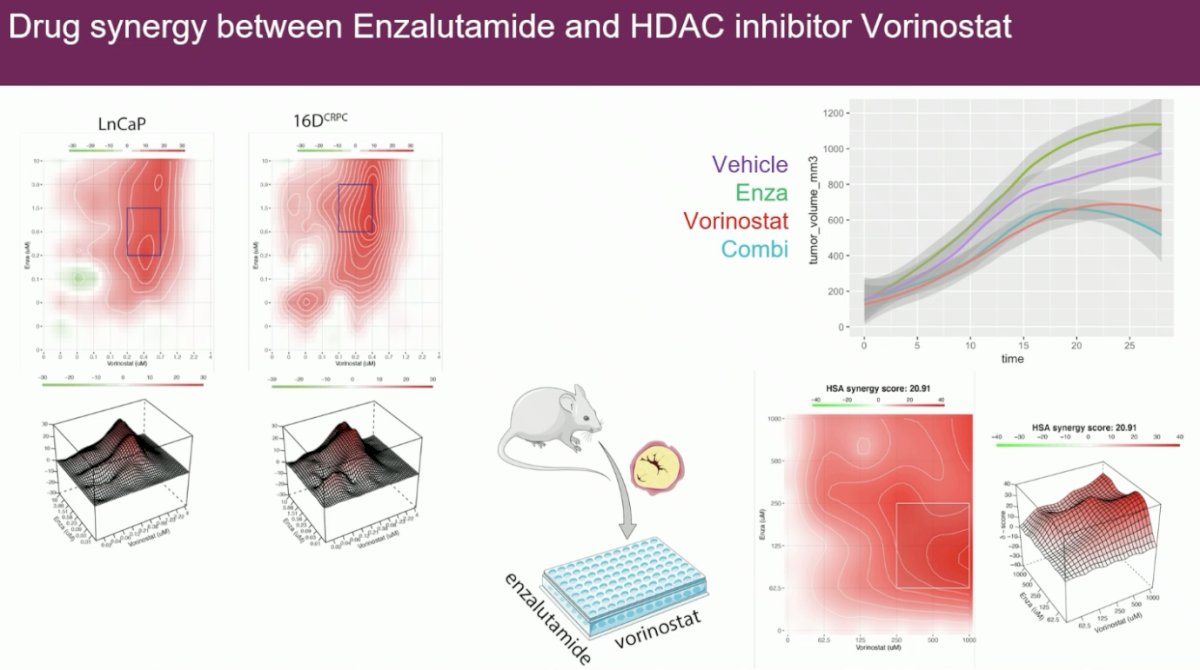
He concluded by noting that epigenetic profiling in mCRPC reveals epigenetics-based biomarkers for response prediction and new synergistic drug combinations.
Presented by: Wilbert Zwart, MD, Professor, Functional Genomics in Oncology, Eindhoven University of Technology, Eindhoven Area, Netherlands
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Linder S, van der Poel HG, Bergman AM, Zwart W, Prekovic S. Enzalutamide therapy for advanced prostate cancer: efficacy, resistance and beyond. Endocr Relat Cancer. 2018; 26(1):R31-52.
- Linder S, Hoogstraat M, Stelloo S, et al. Drug-Induced Epigenomic Plasticity Reprograms Circadian Rhythm Regulation to Drive Prostate Cancer toward Androgen Independence. Cancer Discov. 2022; 12(9):2074-97.


