(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the presentation of the trial in progress 1662. Dr. Qian Qin presented HARMONY: A phase II study of niraparib/abiraterone acetate plus prednisone for Hispanic/Latino and non-Hispanic Black patients with metastatic hormone-sensitive prostate cancer (mHSPC) and deleterious homologous recombination repair alterations (HRRa).
Up to 30% of patients with metastatic castration-resistant prostate cancer (mCRPC) harbor alterations in genes associated with homologous recombination repair (HRR), making them susceptible to poly (adenosine diphosphate-ribose) polymerase (PARP) inhibition. Additionally, PARP1 has been found to regulate both androgen receptor (AR) function and response to DNA damage repair (DDR). Niraparib is a potent and highly selective inhibitor of PARP1 and PARP2. Its combination with abiraterone acetate has been studied in the phase II QUEST study1 and the phase III MAGNITUDE study.2 Both demonstrate that Niraparib plus abiraterone has promising efficacy and a manageable toxicity profile in patients with mCRPC and alterations in DDR genes who had progressed on one prior line of AR pathway inhibitors (ARPIs).
However, there is no data comparing this combination in patients with metastatic hormone-sensitive prostate cancer (mHSPC). There is extensive evidence that racial and ethnic disparities in incidence and survival outcomes have been noted among Hispanic Latinos and non-Hispanic Black versus non-Hispanic White patients diagnosed with prostate cancer. Enrollment of racial and ethnic minorities in clinical trials has remained an unmet need. Dr. Qin designed the HARMONY trial to address this challenge. The HARMONY trial (NCT06392841.) was designed to evaluate the efficacy of targeted followed by adaptive approaches in Hispanic Latino and non-Hispanic Black patients with mHSPC and deleterious HRR alterations.
HARMONY is a multicentric, phase 2, open-label trial run through the Hoosier Cancer Research Network in the USA. Participants will receive ADT + Niraparib/Abiraterone acetate plus Prednisone for 24 weeks, after which they proceed to an adaptive approach:
- Patients with a prostate-specific antigen (PSA) > 4.0 ng/mL (Cohort A) have option to continue ADT + Niraparib/Abiraterone acetate plus Prednisone for a maximum of 2 years or stop Niraparib and add docetaxel (6 cycles), followed by standard of care (SOC) treatment
- Patients achieving a PSA ≤ 4.0 ng/mL (Cohort B) will continue ADT + Niraparib/Abiraterone acetate plus Prednisone. At 12 months on treatment, patients achieving a PSA < 0.2 ng/mL have the option to continue ADT + Niraparib/Abiraterone acetate plus Prednisone for maximum 2 years or discontinue all treatment and monitor, with option to start SOC treatment at disease progression.
The complete study design is illustrated below:

The inclusion criteria for this trial are:
- At least 18 years old and self-identified as Hispanic Latino or non-Hispanic Black
- Histologically confirmed prostate adenocarcinoma
- Diagnosed with mHSPC (minimal treatment: bicalutamide ≤ 45 days, ADT +/- AA plus P ≤ 45 days allowed)
- Deleterious HRR alterations including BRCA 1/2, BRIP1, CHEK2, FANCA, PALB2, RAD51B, and/or RAD54L.
- ECOG ≤2
- Evidence of disease per CT or MRI and bone scan (RECIST and PCWG3)
- Adequate organ function
Main exclusion criteria are:
- Prostate cancer histologic variants (predominant neuroendocrine/small cell carcinoma)
- Other treatment in the mHSPC setting
- Symptomatic brain metastases
- Active malignancies (progressing or requiring treatment change in the last 2 years)
The investigators estimated a 5% dropout rate; therefore, 30 Hispanic Latinos and 30 non-Hispanic Black patients need to be enrolled (total n=60) to complete accrual.
The primary endpoint will be a PSA decline to <0.2 ng/mL at 24 weeks and will be evaluated for each ethnic group against a historical rate of 0.5 ng/mL. This design provides 80% power at a 0.1 significance level to determine non-inferiority, with a non-inferiority margin of 0.185. The secondary and exploratory endpoints include overall survival (OS), objective response rate (ORR), progression-free survival (both PSA and radiographic), time to subsequent anti-cancer therapy, and safety. Additionally, key genomic and quality of life correlatives will be included in the final analysis.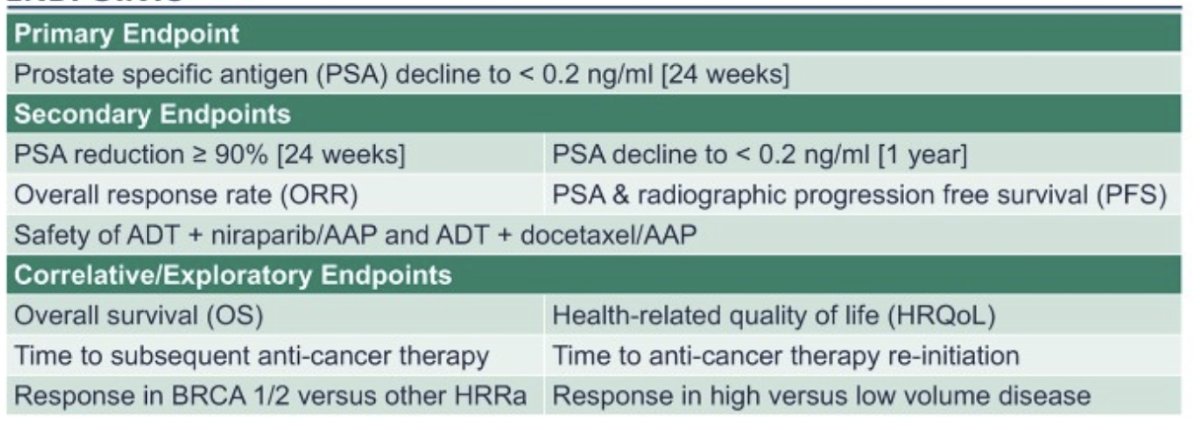
Tissue and blood will be collected to evaluate, circulating tumor cell (CTC) changes with therapy, somatic and germline mutational landscape, and genomic polymorphisms in this NHB and Hispanic Latino population.
Dr. Qin concluded their poster with the following takeaway points:
- HARMONY aims to explore the combination of PARP inhibitors with abiraterone acetate plus prednisone in the front-line setting, using a personalized and adaptive approach to treat metastatic hormone-sensitive prostate cancer.
- This trial will enhance the understanding of genomic alterations and treatment responses among racial and ethnic minorities.
Presented by: Qian Qin MD, Medical Oncologist and Assistant Professor in the Department of Internal Medicine at UT Southwestern Medical Center, Dallas, United States of America.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:- Chi KN, Fleshner N, Chiuri VE, Van Bruwaene S, Hafron J, McNeel DG, De Porre P, Maul RS, Daksh M, Zhong X, Mason GE, Tutrone RF. Niraparib with Abiraterone Acetate and Prednisone for Metastatic Castration-Resistant Prostate Cancer: Phase II QUEST Study Results. Oncologist. 2023 May 8;28(5):e309-e312.
- Chi KN, Rathkopf D, Smith MR, Efstathiou E, Attard G, Olmos D, Lee JY, Small EJ, Pereira de Santana Gomes AJ, Roubaud G, Saad M, Zurawski B, Sakalo V, Mason GE, Francis P, Wang G, Wu D, Diorio B, Lopez-Gitlitz A, Sandhu S; MAGNITUDE Principal Investigators. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2023 Jun 20;41(18):3339-3351. doi: 10.1200/JCO.22.01649. Epub 2023 Mar 23. PMID: 36952634; PMCID: PMC10431499.


