(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the presentation of Poster 1618. Dr. Anis Hamid discussed the clinical prognostic factors within the high-volume subgroup of metastatic hormone-sensitive prostate cancer (mHSPC) in the ENZAMET (ANZUP 1304) study.
ENZAMET (NCT02446405) was an international, open-label, randomized, phase 3 trial conducted at 83 sites across Australia, Canada, Ireland, New Zealand, the UK, and the USA. Participants were males aged 18 years or older diagnosed with metastatic, hormone-sensitive prostate cancer with an Eastern Cooperative Oncology Group performance status score of 0-2. Participants were randomly assigned in a 1:1 ratio to androgen deprivation therapy (ADT) combined with oral enzalutamide (160 mg once daily) or a standard oral non-steroidal antiandrogen (bicalutamide, nilutamide, or flutamide) until clinical disease progression or prohibitive toxicity.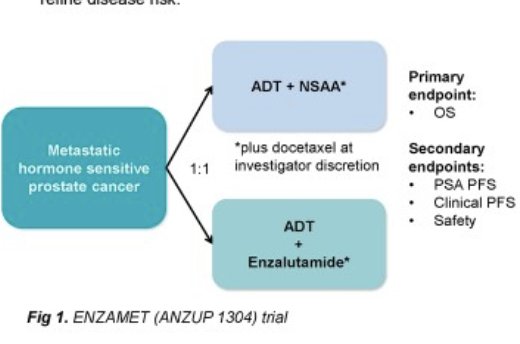
ENZAMET showed that enzalutamide added to ADT with or without docetaxel improves overall survival (OS) versus ADT plus standard non-steroidal anti-androgen (NSAA) (1). Dr Hamid shared that patients with high-volume mHSPC have poorer outcomes than those with low-volume (LV) disease, despite improved OS with enzalutamide in both subgroups. They explored if intrinsic tumour features and metastatic bone burden further refine disease risk within this subgroup and this was the purpose of this post-hoc analysis of ENZAMET.
Dr. Hamid and colleagues did a Post-hoc analyses of T-stage, Gleason score, visceral metastases, and bone burden, were performed in the following subgroups:
- M1(synchronous)-HV
- M0(metachronous)-HV
- M1-LV
They defined high bone burden as the upper quartile of alkaline phosphatase at trial enrolment and the bone burden analyses excluded patients with visceral metastases.
The Endpoints of this post-hoc analysis were OS, prostate cancer-specific survival (PCSS), and PSA progression-free survival (PSA-PFS). They used univariable Cox models to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs).
A total of 439 patients with M1-HV (Synchronous HV) disease were identified in this post-hoc analysis of ENZAMET. Of these, 179/344 (52%) had high bone burden (ALP>150 IU/L), 93% had ≥ 4 bone metastases and 97% had more or equal than one bone metastasis outside the pelvis/vertebrae. Baseline patient characteristics are detailed below: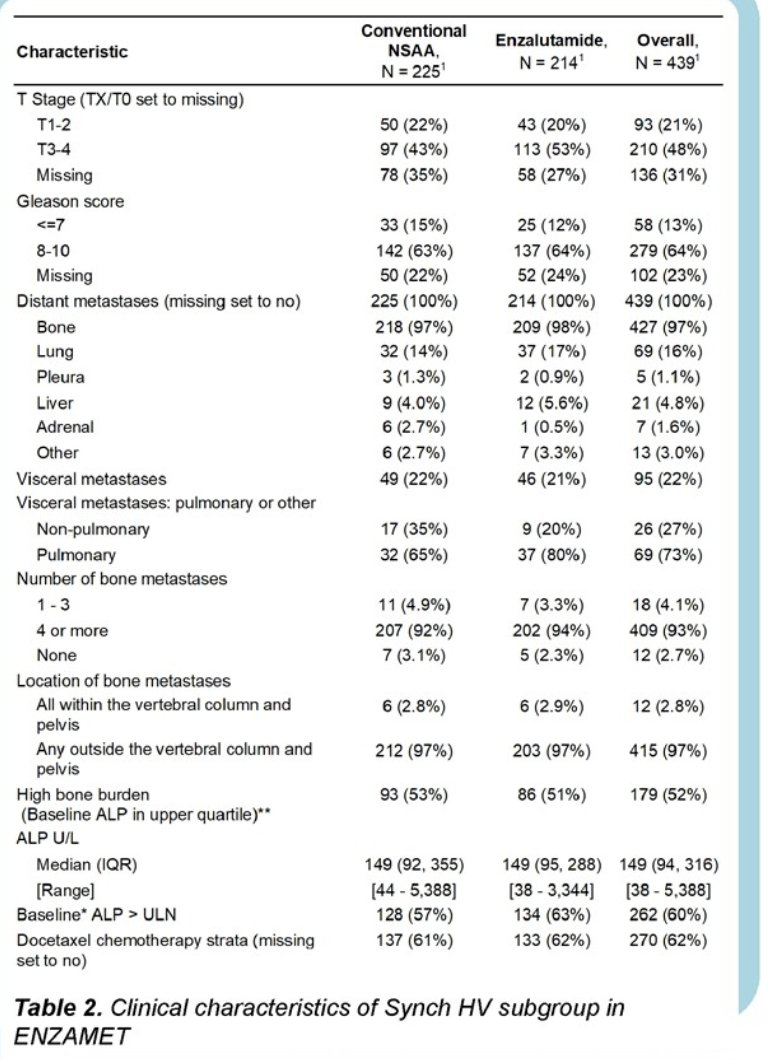
In patients with synchronic HV disease high bone burden was associated with shorter OS (HR 1.64, 95% CI 1.23-2.2, p<0.001). The 5-year OS for those with high bone burden vs. those without it was 43% vs. 59%. High Gleason score (Gleason 8-10) was associated with shorter OS (HR 1.80,95% CI 1.14-2.83, p=0.01), but T-stage (T3/T4 vs. T1/T2) was not associated with shorter OS (HR 1.2, 95% CI 0.86-1.69, p=0.29) neither visceral metastasis (HR 1.0, 95% CI 0.74-1.36, p=1.0)
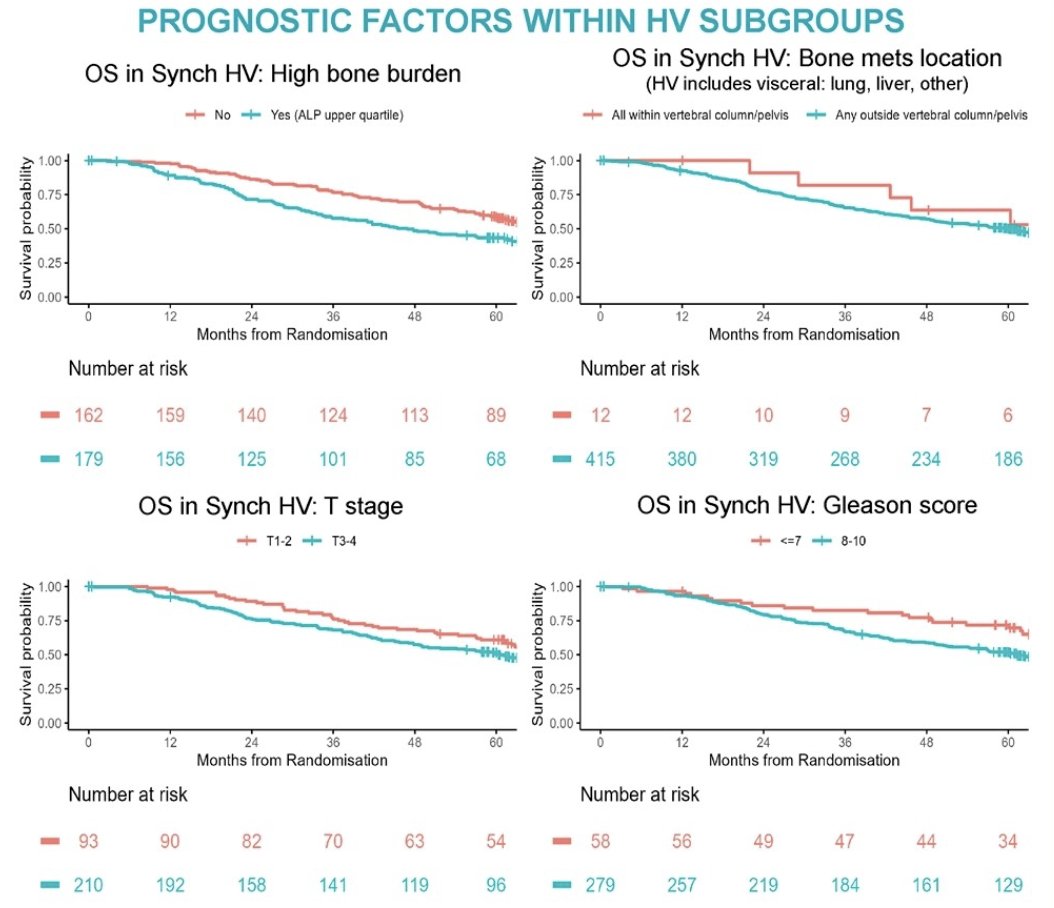
Bone metastases limited to vertebral column and pelvis (despite HV status by presence of visceral metastasis were associated with greater 5-year OS compared with extensive bone metastases (OS HR 1.41, p=0.449, 5-year OS: 64% vs 50%);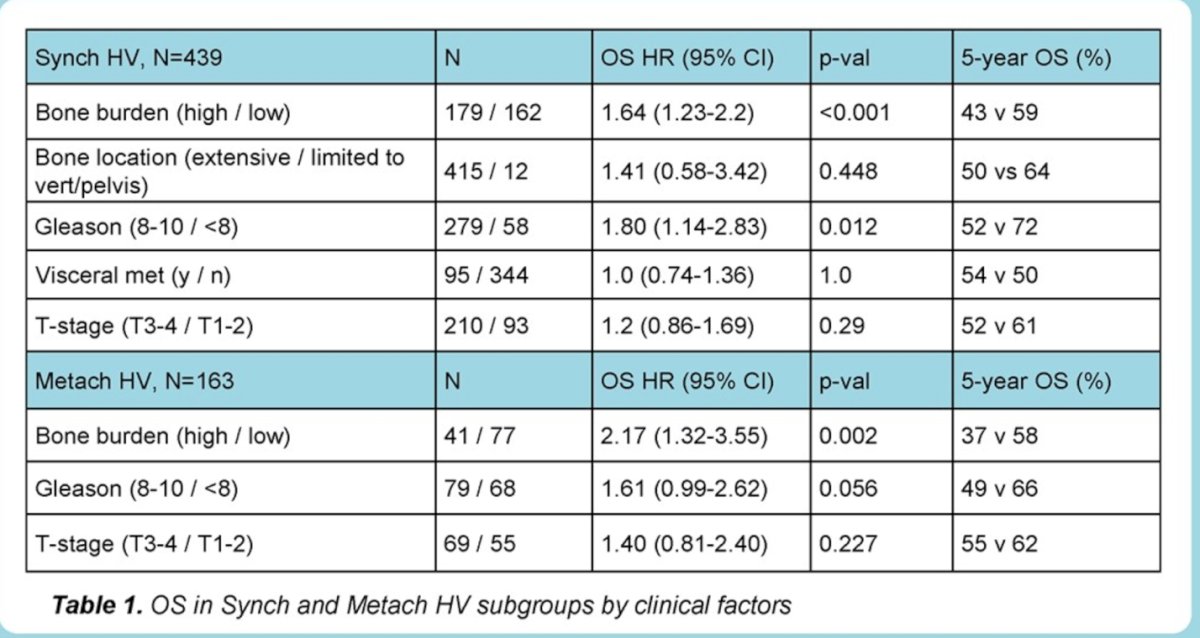
Patients with M1-HV receiving ADT+Enzalutamide did not have significantly different OS by treatment with docetaxel within bone burden subgroups (low/high), in non-randomized patients.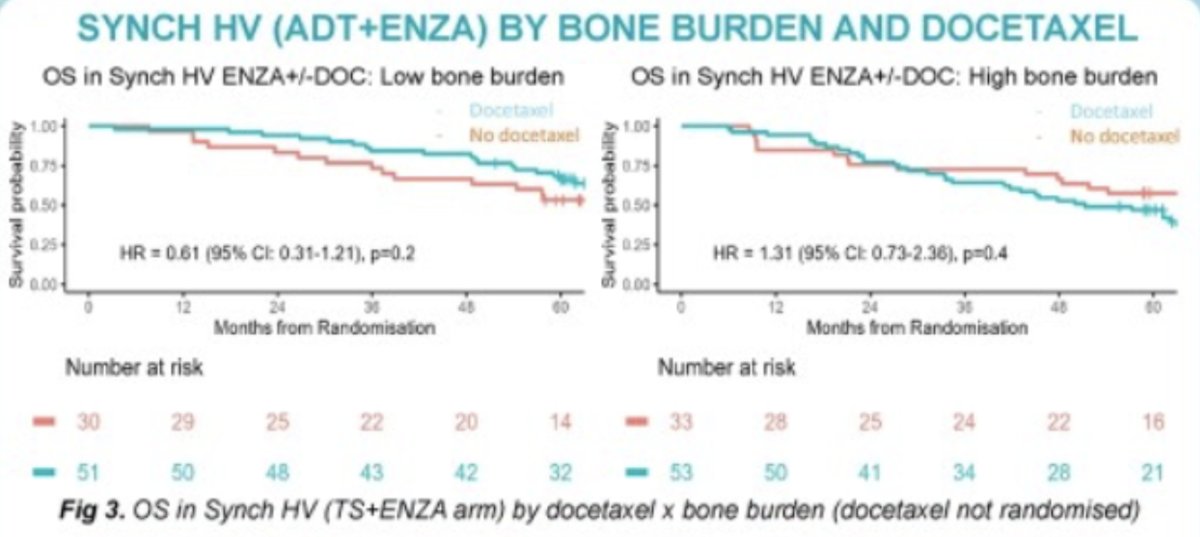
Similar findings were seen for PCSS and for PSA-PFS, However, Gleason score was not significantly associated with worse PSA-PFS in patients with M1-HV.
In patients with M0-HV (metachronous-HV), high bone burden (HR 2.17, 95% CI 1.32-3.55, p=0.002) and high Gleason score (HR 1.61, 95% CI 0.99-2.62, p=0.056) were associated with poorer OS and also PCSS.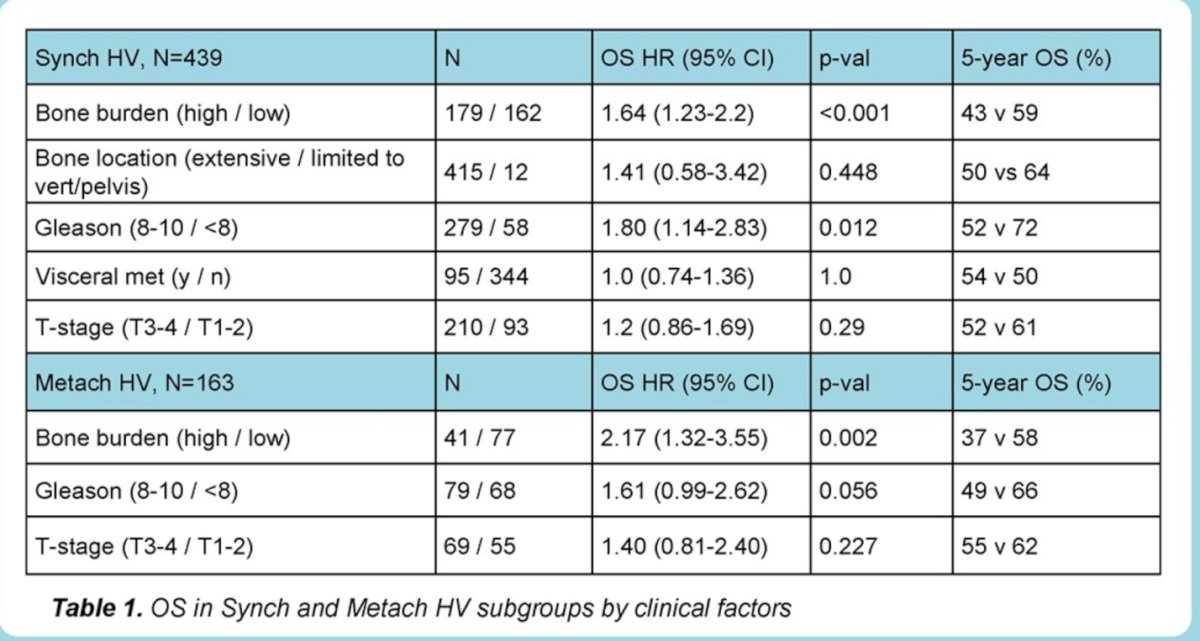
Interestingly no factors were identified as predictors of OS or PCSS in M1-LV including bone metastasis location, Gleason score, or T-stage. Interestingly, in the LV- subgroup presence of ≥ 4 bone metastases were associated with an improved 5-year OS compared to non-visceral HV disease (5-year OS 69% vs. 58%).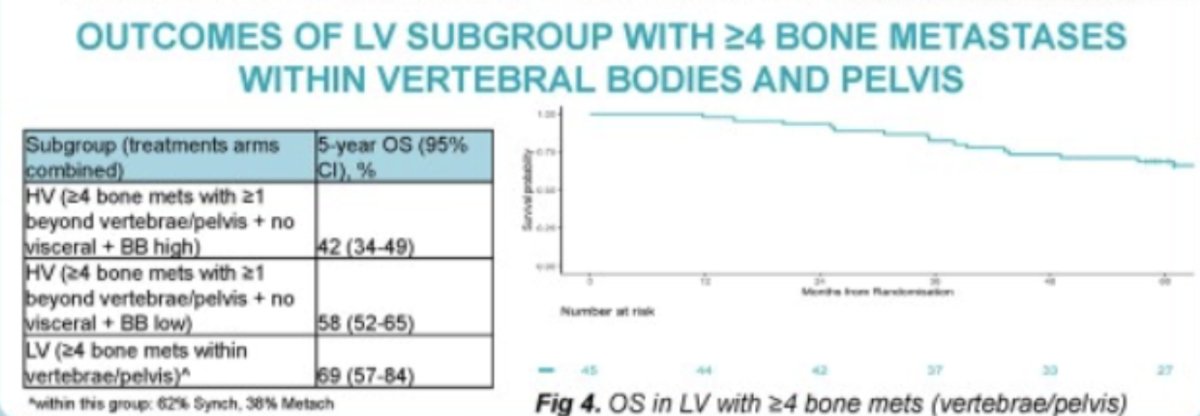
Dr. Hamid concluded his presentation with the key following messages:
- In this post-hoc analysis of ENZAMET, high ALP was identified as a possible indicator of bone burden in patients without visceral metastasis.
- ALP was prognostic in patients with HV-mHSPC in the ENZAMET trial for PSA-PFS, PCSS, and OS.
- High GS was associated with poorer outcomes in HV-Synchronic mHSPC
- Further stratification of highest-risk patients with mHSPC may help define cohorts for further therapy intensification
- LV synchronic mHSPC disease with ≥ 4 bone metastasis was associated with better OS compared with non-visceral HV mHSPC disease.
- These data may help with identification of pts with poorest prognosis mHSPC for future studies of further therapy intensification.
Presented by: Anis A. Hamid, MBBS, GU Oncology, Department of Medical Oncology, Peter MacCallum Cancer Centre, and University of Melbourne, Melbourne, Victoria, Australia. Genitourinary Medical Oncologist at Memorial Sloan Kettering Cancer Center, NY, United States of America.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:
- Sweeney CJ, Martin AJ, Stockler MR, Begbie S, Cheung L, Chi KN, Chowdhury S, Frydenberg M, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCaffrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W, Pook DW, Reaume MN, Sandhu SK, Tan A, Tan TH, Thomson A, Vera-Badillo F, Williams SG, Winter D, Yip S, Zhang AY, Zielinski RR, Davis ID; ENZAMET trial investigators and Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023 Apr;24(4):323-334.


