(UroToday.com) The 2024 ESMO annual meeting included a highlights session, featuring a presentation by Dr. Ursula Vogl discussing highlights of the ESMO 2024 prostate cancer sessions. The outline of prostate cancer highlights to guide Dr. Vogl’s discussion is summarized in the following figure:

Dr. Vogl started by discussing “Prostate cancer efficacy results from a randomized phase 3 evaluation of transdermal estradiol versus LHRH agonists for androgen suppression in M0 prostate cancer.” Transdermal estradiol achieves equivalent castration rates to LHRH analogues without the excess cardiovascular morbidity or mortality reported with oral estrogen. The potential benefits of transdermal estradiol compared with LHRH analogues include improved bone mineral density, more favorable metabolic profiles, and better quality of life over 6 months of ADT, although with increased likelihood of gynecomastia. This was an open-label, randomized phase 3, non-inferiority comparison of LHRH agonist versus transdermal estradiol patches: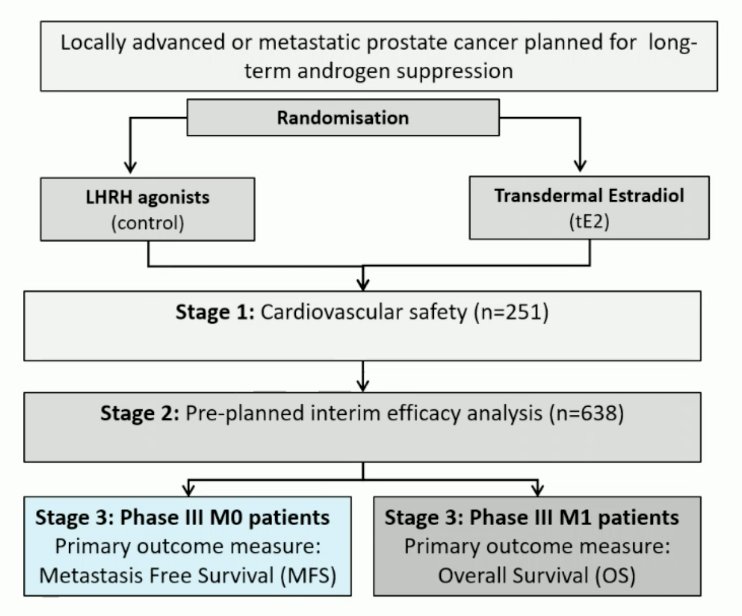
The trial design is as follows: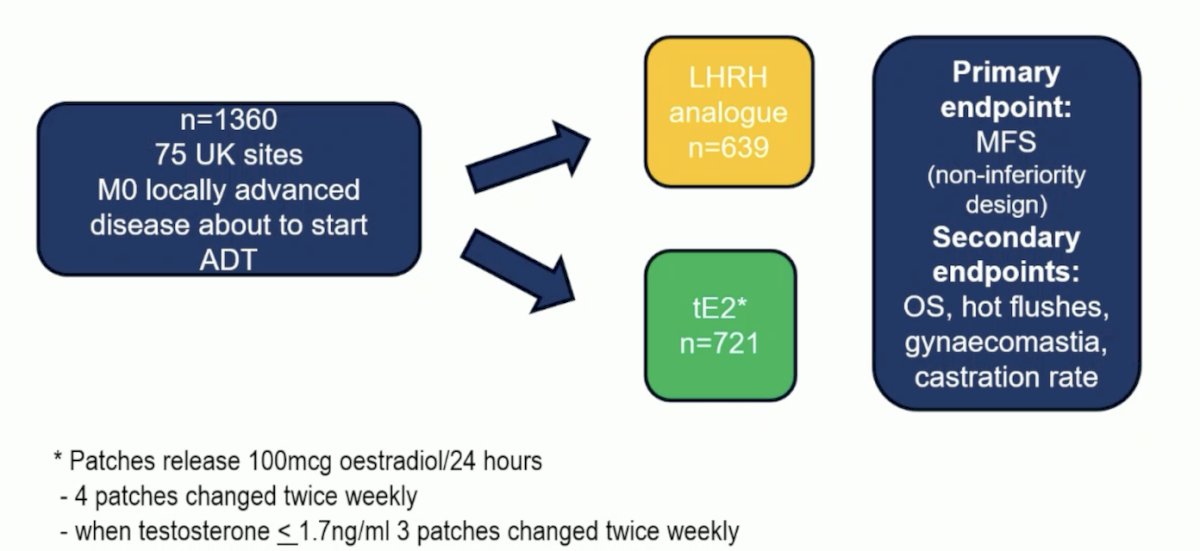
The LHRH agonist 3-year metastasis-free survival rate was 87% (giving a target non-inferiority HR of 1.31). Transdermal estradiol 3-year metastasis-free survival was 86% HR 0.96 (95% CI 0.81-1.14) in favor of transdermal estradiol, excluding a 2% reduction in metastasis-free survival: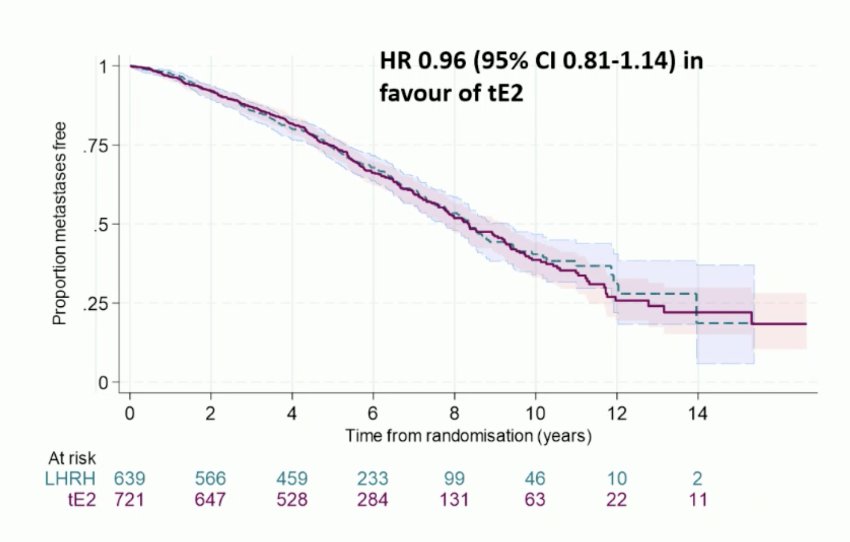
Overall survival was HR 0.89 (95% CI 0.74-1.07) in favor of transdermal estradiol: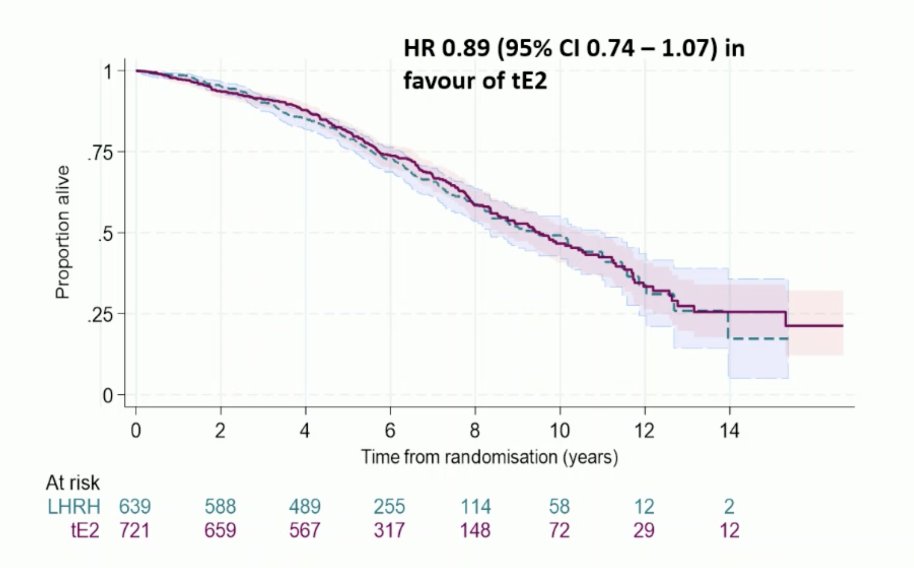
Sustained castration rate was defined as testosterone ≤1.7 nmol/L over 1 year; n = 1,066), with transdermal estradiol use confirmed as estradiol ≥250 pmol/L; 85% for both groups. LHRH agonist versus transdermal estradiol any grade adverse events included gynecomastia 42% versus 85% and hot flushes 89% versus 44%, respectively. Dr. Vogl notes that in her concluding statements, Dr. Langley emphasized that transdermal estradiol should be a standard-of-care ADT option in M0 disease. However, Dr. Vogl notes that there are several open questions before transdermal estradiol can be routinely used:
- Transdermal estradiol in mHSPC has not yet been reported
- How do we deal with the gynecomastia?
- Will patients be compliant with the patch application?
- What happens with testosterone recovery?
- Does the better bone mineral density translate to less bone fractures?
- What are the metabolic changes in the long term?
- Is there less cognitive impairment?
Next, Dr. Vogl discussed the ARANOTE trial in the mHSPC setting. Indeed, the management of mHSPC is an evolving systemic treatment paradigm: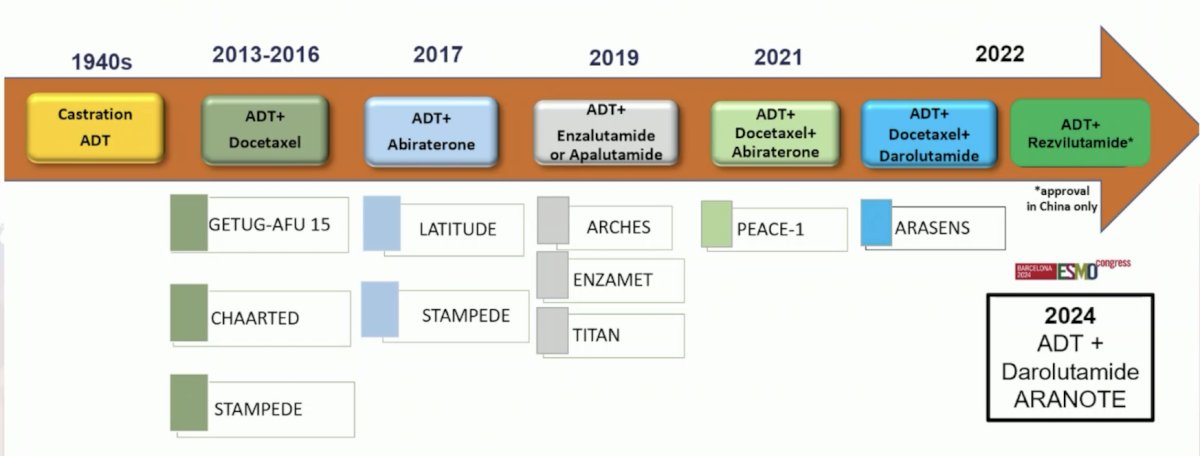
Eligible patients for ARANOTE had mHSPC by conventional imaging, an ECOG performance status of 0–2, and started ADT ≤ 12 weeks. Patients were randomized 2:1 to darolutamide 600 mg twice daily or placebo, each with ADT. The primary endpoint was radiological progression-free survival, and secondary endpoints included overall survival, time to initiation of subsequent anticancer therapy, time to CRPC, time to PSA progression, time to pain progression, and safety. The trial design for ARANOTE is below: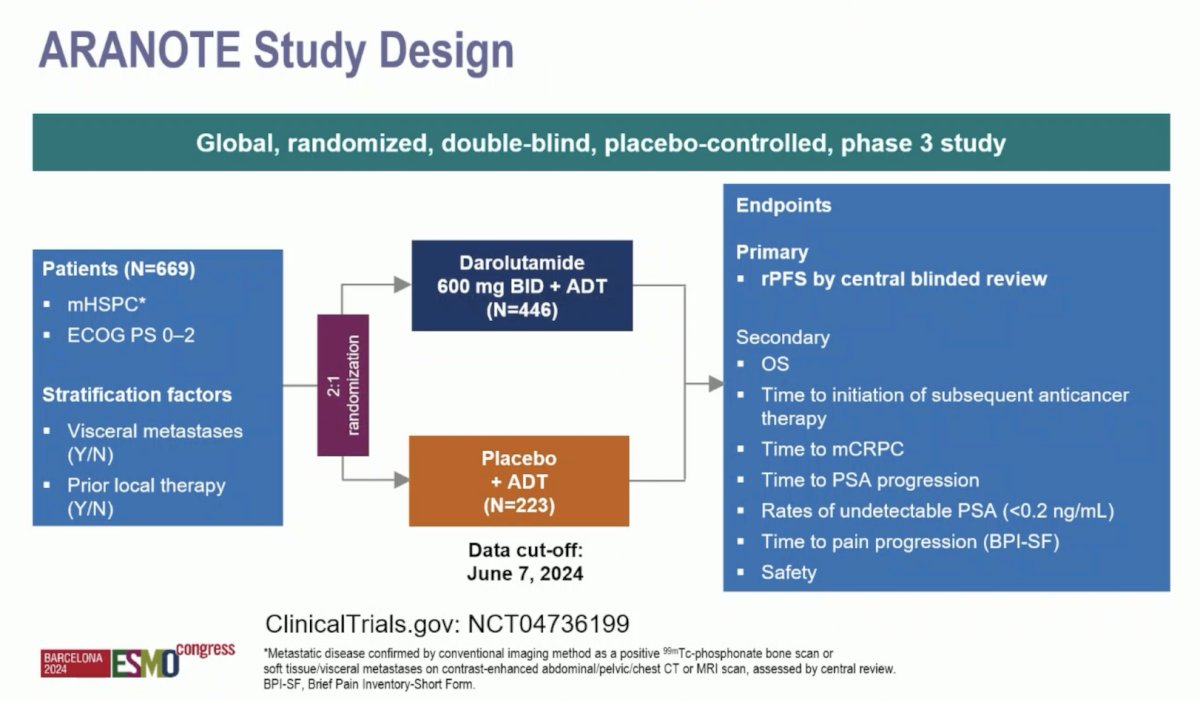
Dr. Vogl notes that all other ADT + ARPI mHSPC trials, except ARCHES, had overall survival as the primary or at least the co-primary endpoint. A total of 669 patients were randomized (darolutamide, n = 446; placebo, n = 223). The median age was 70 years, 31% were Asian, 9.7% were Black, the median PSA at baseline was 21.3 ng/mL, and 71% had high-volume mHSPC: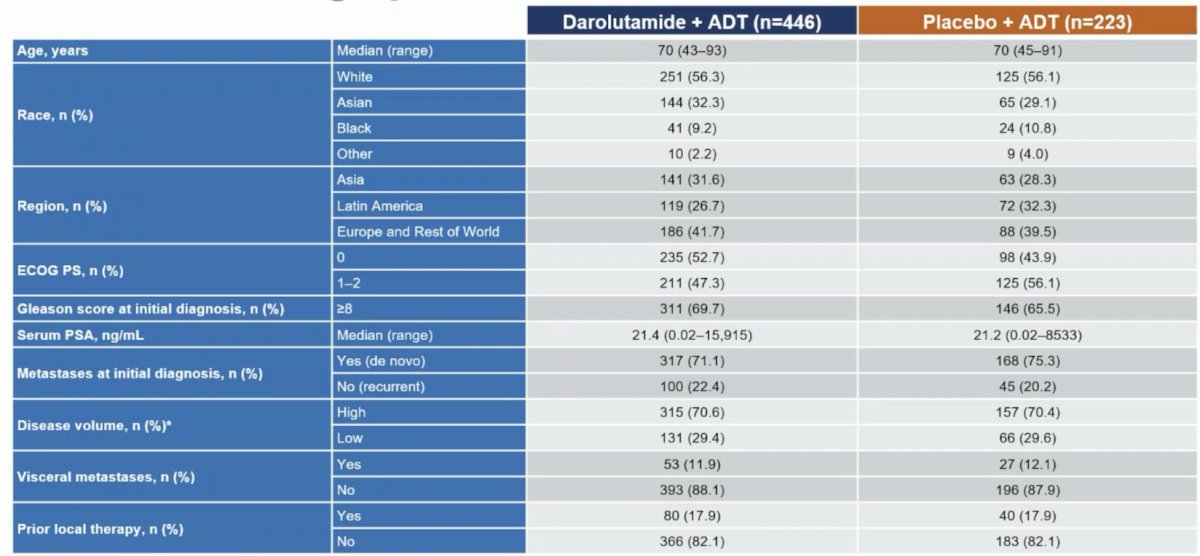
At the primary data cutoff of June 7, 2024, darolutamide + ADT significantly improved radiological progression-free survival versus placebo + ADT (HR 0.54, 95% CI 0.41–0.71):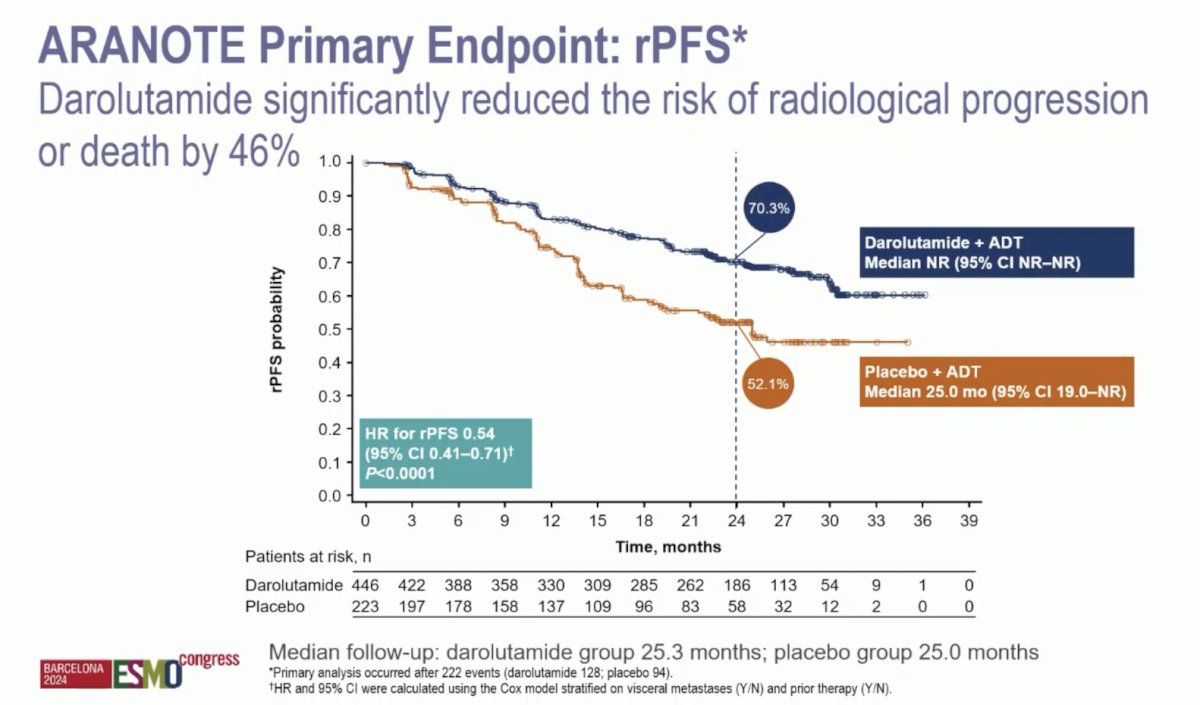
There were also consistent radiographic progression-free survival benefits observed across prespecified subgroups, including patients with high- and low-volume mHSPC: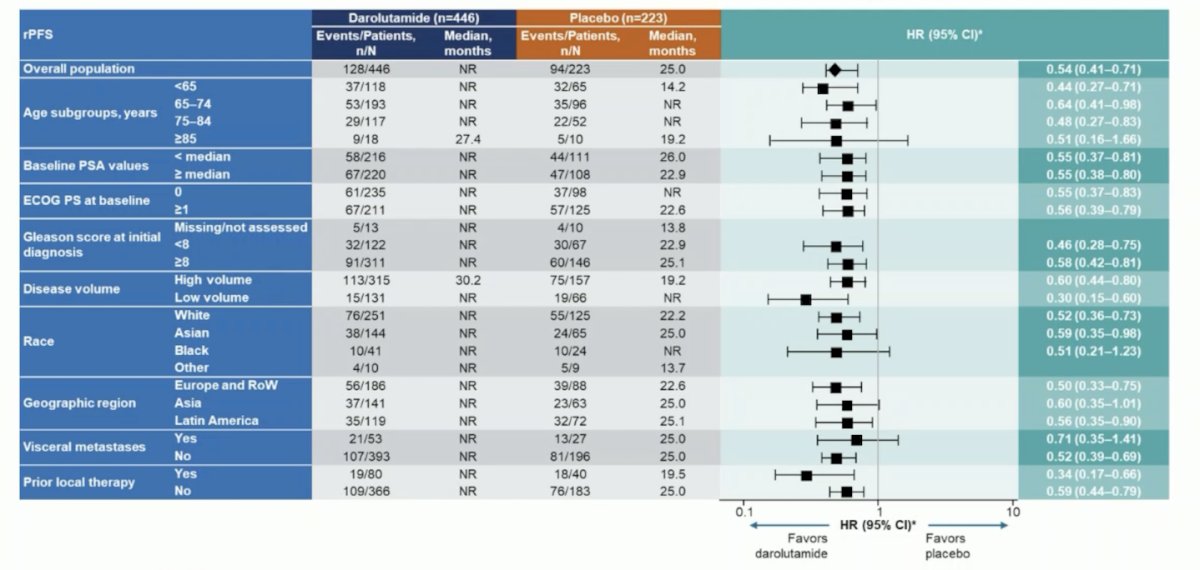
Importantly, darolutamide showed a clinical benefit across all secondary endpoints: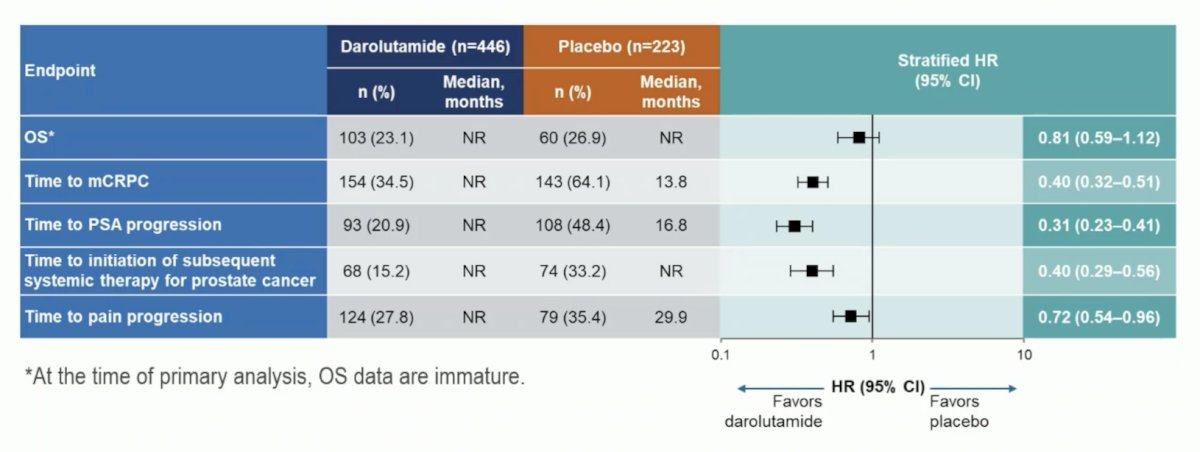
Incidences of treatment-emergent adverse events were low and similar between groups, and treatment discontinuations due to treatment-emergent adverse events were lower in patients receiving darolutamide versus placebo (6.1% vs 9.0%):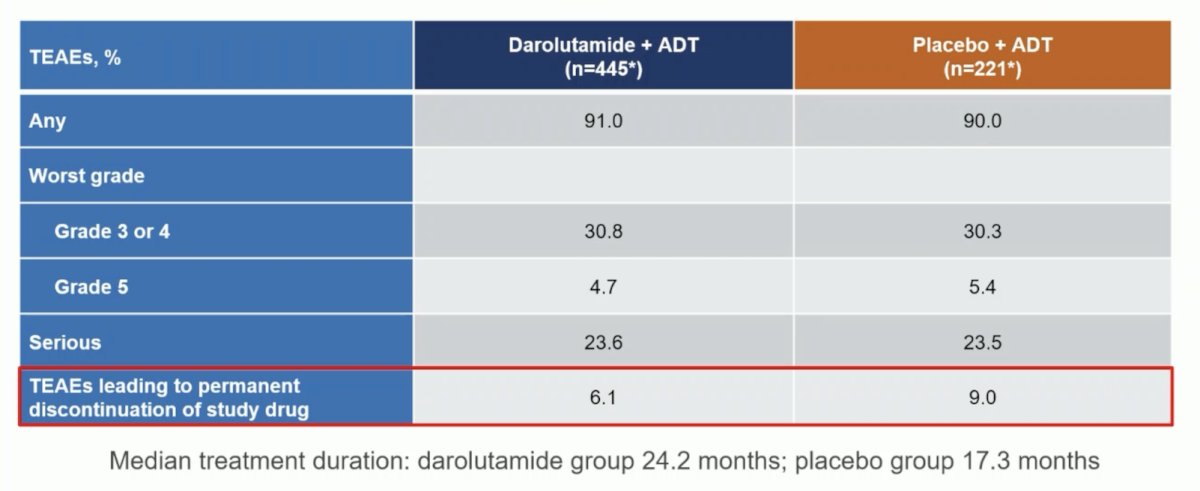
The following table shows that treatment-emergent adverse events associated with androgen receptor pathway inhibitors were generally similar between treatment groups; fatigue was less common in the darolutamide + ADT group versus placebo + ADT: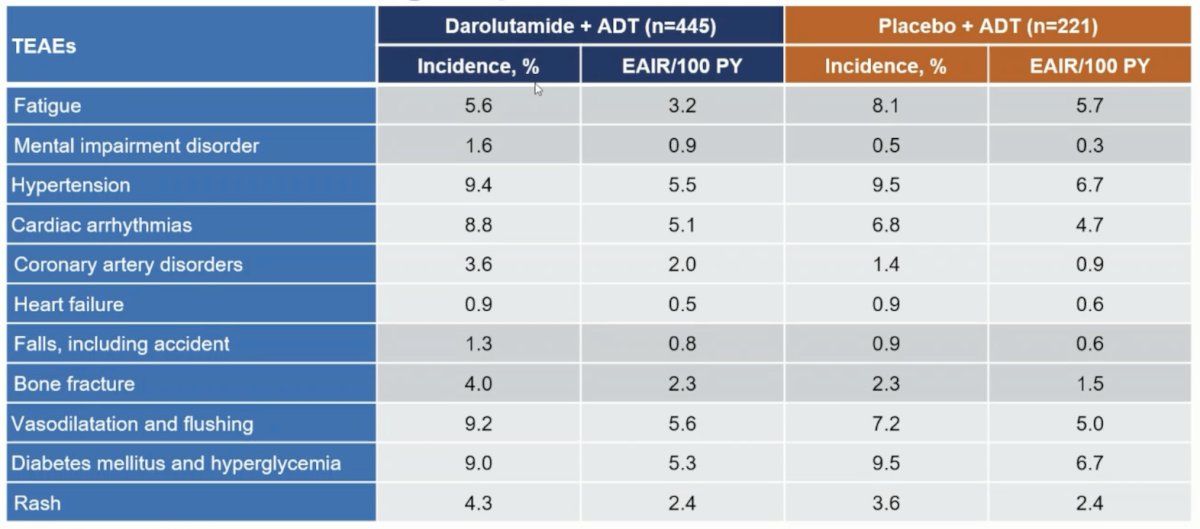
Secondary to a slightly increased risk of bone fractures in the treatment arm, Dr. Vogl notes that it is fundamental to evaluate bone-protecting agents in a prophylactic dosing in mHSPC patients with combination hormonal therapies. Dr. Vogl concluded with similar conclusions to Dr. Saad’s conclusions after his ARANOTE presentation:
- Darolutamide + ADT significantly improved radiographic progression-free survival in patients with mHSPC
- Darolutamide showed a benefit across all secondary endpoints
- Darolutamide had a favorable safety profile
- Based on these results for ARANOTE, darolutamide + ADT without docetaxel should become an additional standard of care for mHSPC.
The second trial in the mHSPC space discussed was “Adding metformin to ADT for patients with mHSPC: Overall survival results from the multi-arm, multi-stage randomized platform trial STAMPEDE.” There have been several randomized phase 2/3 trials investigating the used of metformin in prostate cancer: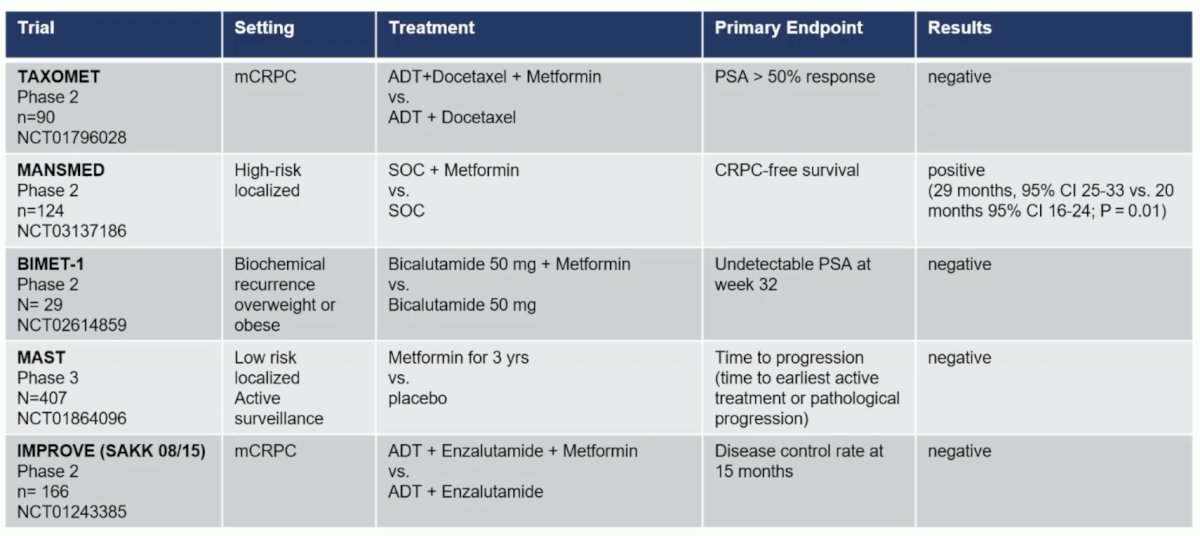
In the STAMPEDE trial, non-diabetic patients with mHSPC were randomly allocated 1:1 to standard of care or standard of care + metformin. Standard of care included ADT ± radiotherapy ± docetaxel ± androgen receptor pathway inhibitor. The primary outcome was overall survival. The trial design is as follows: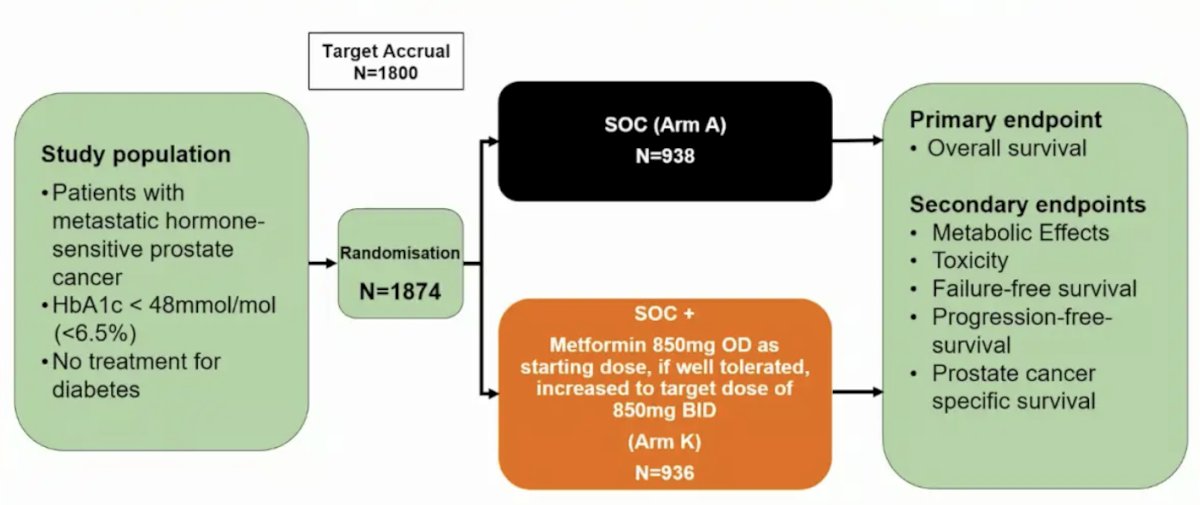
There were 1,874 patients with mHSPC randomized September 2016 through March 2023. The arms were well balanced, with a median age of 69 years (IQR 63-73), median PSA of 84 ng/ml (IQR 24-352), and de novo metastatic disease in 1,758 (94%) patients versus relapsed in 116 (6%). Planned standard of care included 82% of patients receiving docetaxel and 3% receiving androgen receptor pathway inhibitor: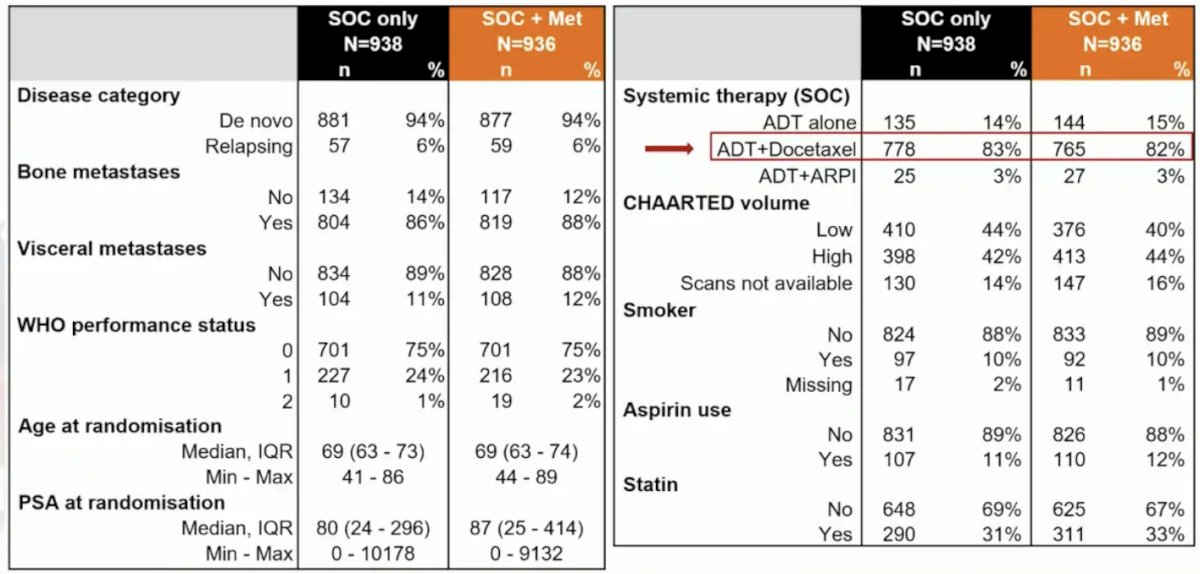
After a median follow-up of 60 months, the HR for overall survival between arms was 0.91 (95% CI 0.80-1.03; p = 0.148). The median overall survival was 63.1 (95% CI 57.6-68.7) and 69.1 (95% CI 62.7-73.2) months in the standard of care and standard of care + metformin arms, respectively: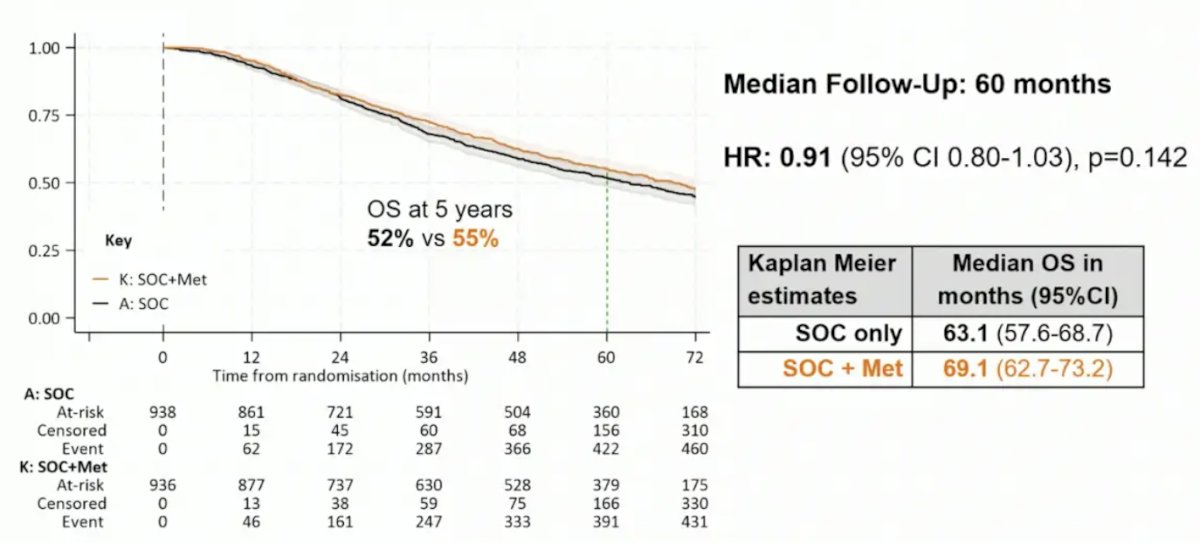
In patients with high versus low volume disease (CHAARTED definition), HR was 0.79 (95% CI 0.66-0.93; p = 0.006) and 1.00 (95% CI 0.79-1.26; p = 0.992), respectively, with an interaction p-value of 0.086: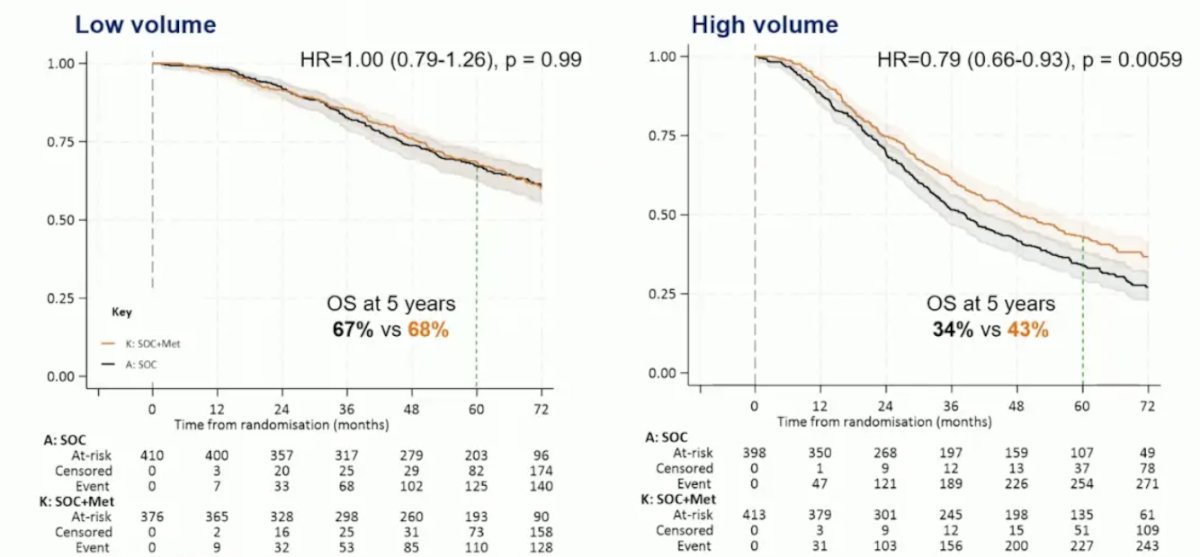
Metabolic parameters that improved significantly with metformin included reduced weight gain (-2.5, 95% CI -3.6 to -1.4), fasting glucose (-0.17, 95% CI -0.29 to -0.05), and HbA1c (-1.0, 95% CI -1.6 to -0.5). Additionally, the metformin arm decreased total (-0.16, 95% CI -0.29 to -0.03) and LDL cholesterol (-0.17, 95% CI -0.29 to -0.05):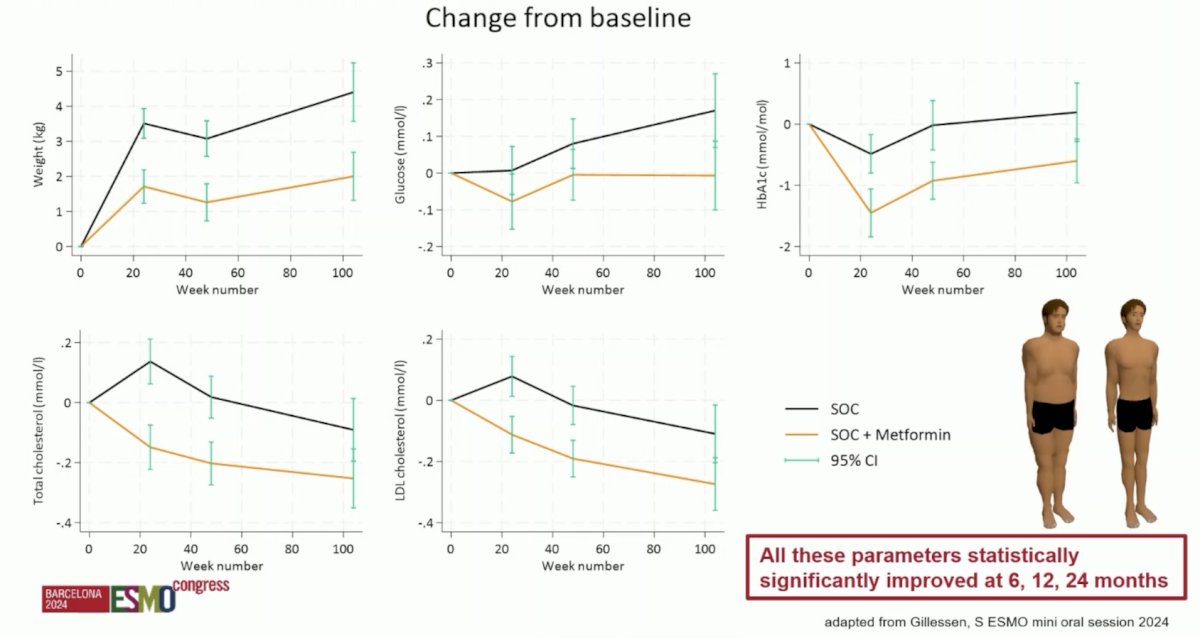
Adverse events ≥ grade 3 were reported in 52% and 57% in the standard of care and standard of care + metformin arms, respectively. Gastrointestinal adverse events increased with metformin: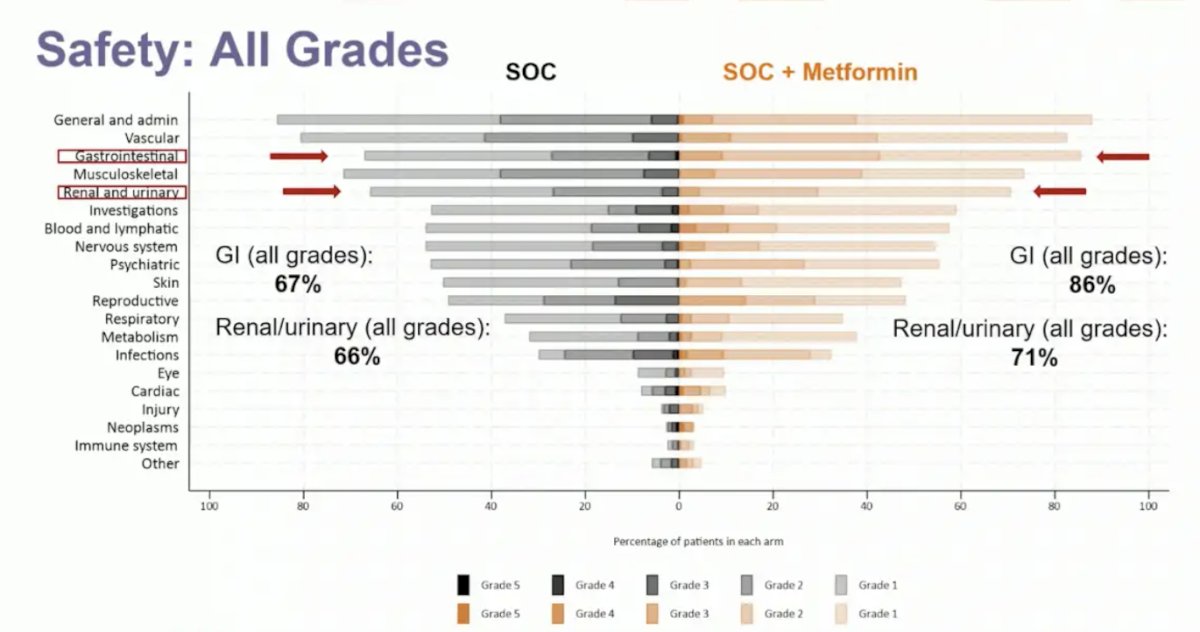
Dr. Vogl provided the following summary statements for STAMPEDE Arm K assessing metformin in mHSPC patients:
- There is no clear evidence that adding metformin to the standard of care increases overall survival in unselected patients with mHSPC
- There is some evidence of overall survival benefit in the pre-specified, but not pre-powered subgroup with high volume disease that needs further evaluation.
- Most metabolic parameters improved significantly with metformin irrespective of disease volume.
- Further investigation is warranted in ADT + ARPI combination in mHSPC (in STAMPEDE there was only 3% of patients that received ADT + ARPI)
Dr. Vogl then moved to the mCRPC space, where there has also been developing advances of treatment: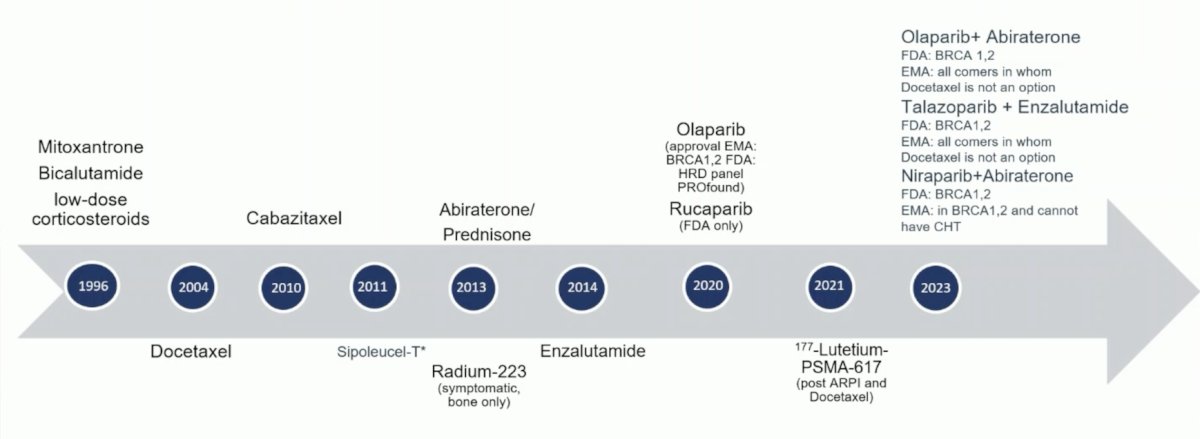
First, Dr. Vogl discussed “Radium-223 + enzalutamide in asymptomatic or mildly symptomatic patients with bone mCRPC: First results of EORTC-GUCG 1333/PEACE-3. PEACE-3)” is a prospective phase III trial of mCRPC patients with bone metastases who were asymptomatic or mildly symptomatic. Eligible patients had not received prior enzalutamide or Radium-223 and had no known visceral metastases. Prior treatment with abiraterone and docetaxel in the hormone-sensitive setting was permissible. Study participants underwent 1:1 randomization to:
- Experimental arm: Radium-223 55 kBq/kg intravenously every 4 weeks for 6 cycles + enzalutamide 160 mg orally once daily
- Control arm: Enzalutamide 160 mg orally once daily
The primary endpoint was radiographic progression-free survival, and key secondary endpoints included:
- Safety
- Overall survival
- Time to next treatment
- Time to pain progression
- Time to first symptomatic skeletal event
The trial design for PEACE-3 is as follows: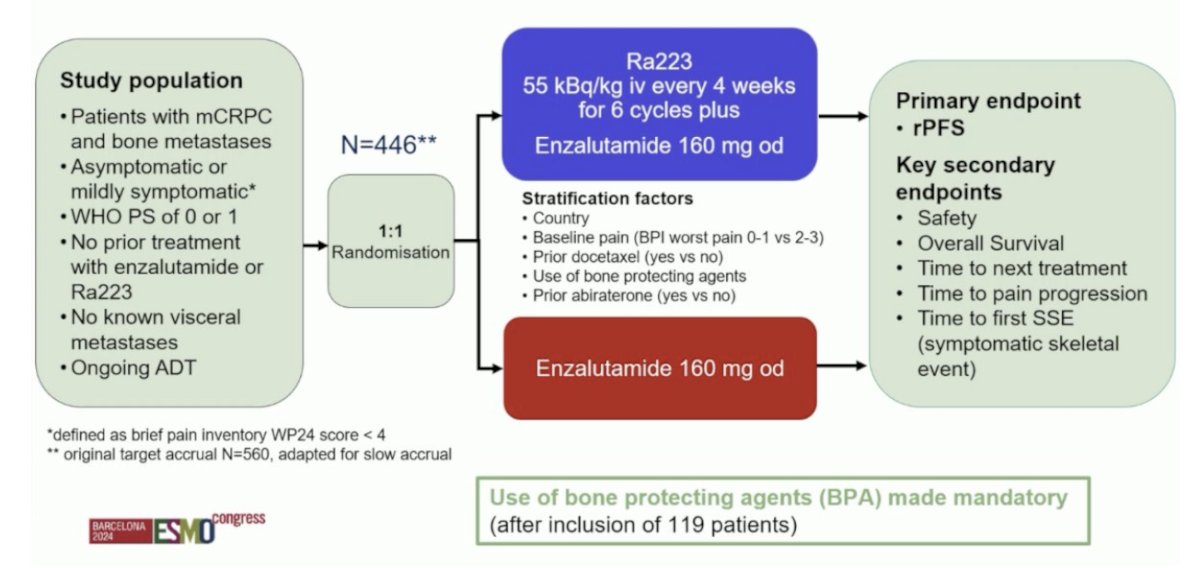
Given the increased rate of skeletal fractures reported in the ERA-223 trial, a study amendment was introduced following the enrollment of the initial 119 patients to mandate the use of bone-protecting agents. Between November 2015 and March 2023, 446 patients were enrolled from 12 countries, with a median study follow-up of 42.2 months. The median patient age was 70 years, and the median PSA was 23–25 ng/ml. Approximately, 30% of patients had received prior docetaxel in the hormone-sensitive setting, and only 2–3% had received prior abiraterone: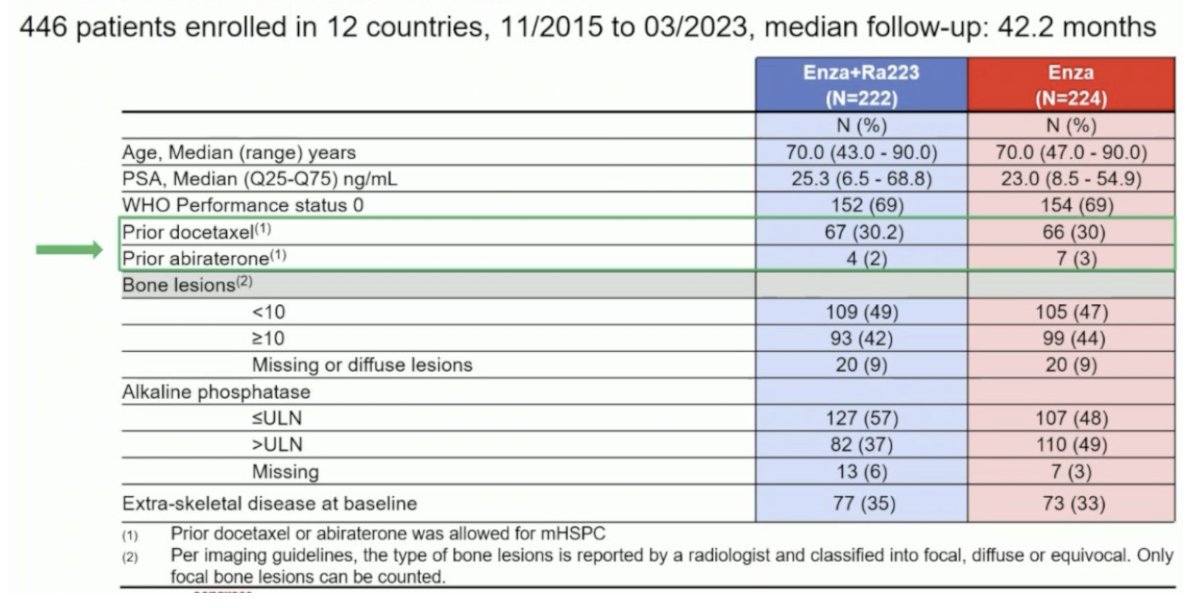
The addition of Radium-223 to enzalutamide was associated with significant improvements in the primary endpoint of radiographic progression-free survival (median: 19.4 versus 16.4 months; HR: 0.69, 95% CI: 0.54–0.87, p = 0.0009). At 24 months, 45% of patients in the Radium-223 combination arm were free of radiographic progression, compared to 36% of patients in the enzalutamide monotherapy arm: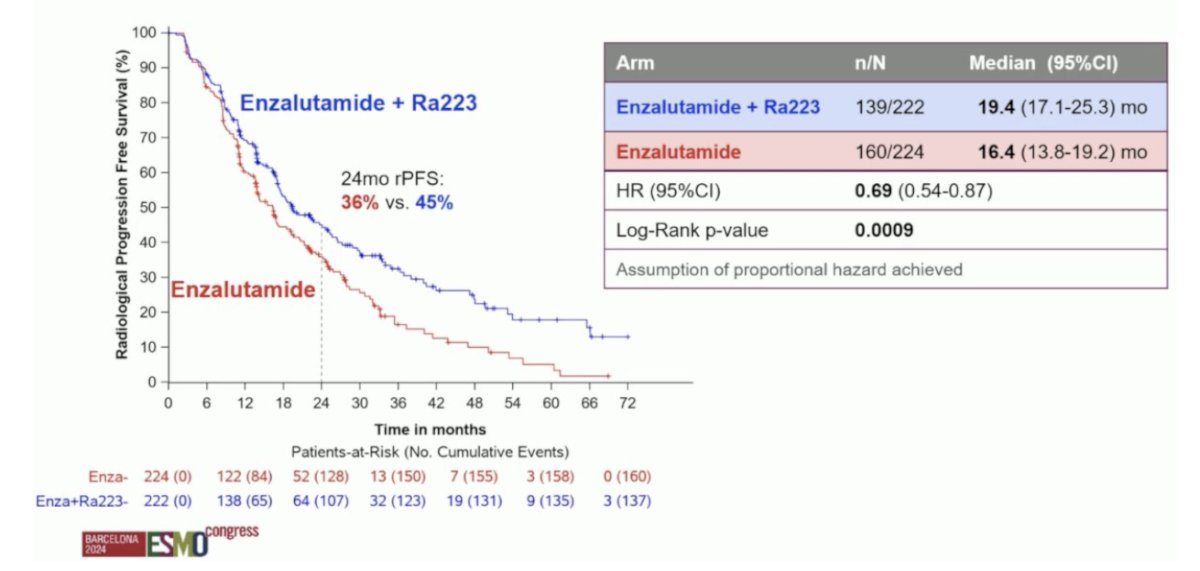
An overall survival benefit was observed with a combination of Radium-223 + enzalutamide. The median overall survival was 42.3 months versus 35 months for enzalutamide monotherapy (HR: 0.69, 95% CI: 0.52–0.90, p=0.0031). While the p-value was below the pre-specified α threshold of 0.0034, there was evidence of non-proportional hazards (i.e., the curves cross during initial follow-up). Given this plus the lack of unequivocal significance for restricted mean survival time sensitivity analysis, the study will continue to the final overall survival analysis: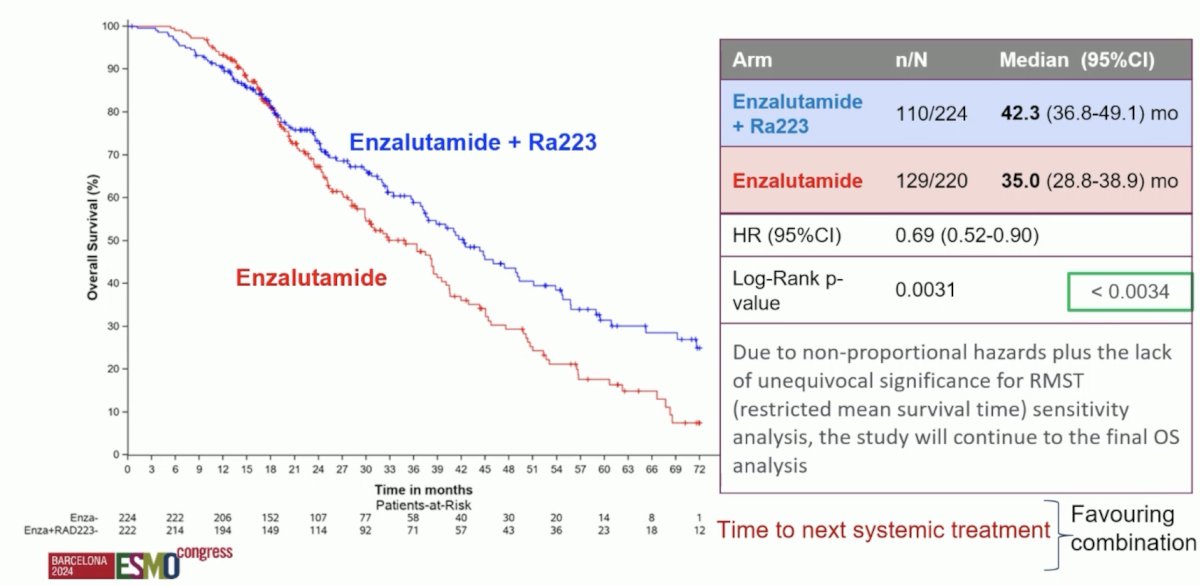
From a safety standpoint, grade ≥3 drug-related adverse events were observed in 28% and 19% of patients in the experimental and control arms, respectively. Deaths due to adverse events were observed in 3% and 2% of patients, respectively, none specifically related to the drugs used: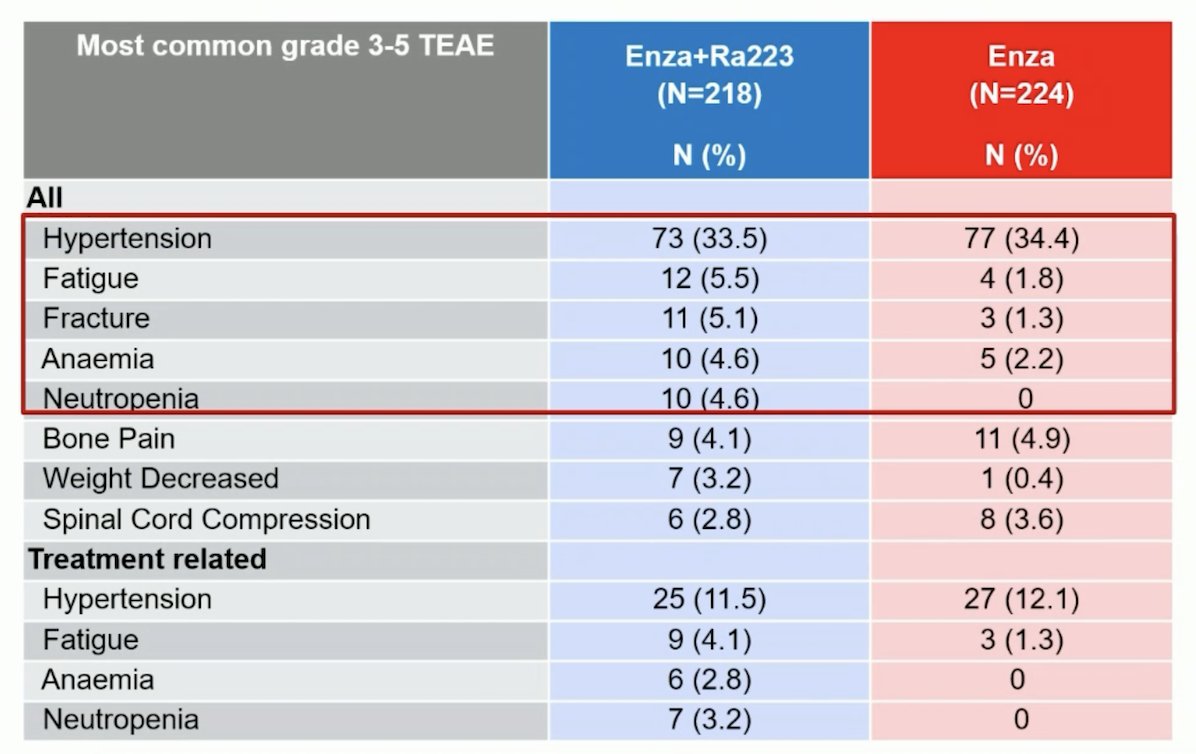
At the time of her presentation, Dr. Gillessen stated that “these results support the combination of enzalutamide + Radium-223 (plus a bone protecting agent) as a potential new first-line mCRPC treatment option for patients with prostate cancer and bone metastases who have not received a prior ARPI.” In Dr. Fizazi’s discussant presentation he noted that “yes, this is practice-changing data, with the caveats about long-term overall survival and missing osteonecrosis of the jaw data. Moreover, most patients (depending on the region) have received an ARPI already in mHSPC.”
Finally, Dr. Vogl discussed “SPLASH: Efficacy of 177Lu-PNT2002 in PSMA-positive mCRPC following progression on an androgen-receptor pathway inhibitor.” The role of 177Lu-PSMA-617 in mCRPC has been previously evaluated post docetaxel +/- an ARPI in the phase 3 VISION trial assessing 177Lu-PSMA-617 versus protocol permitted standard of care1 and the phase 2 TheraP trial assessing 177Lu-PSMA-617 versus cabazitaxel:2
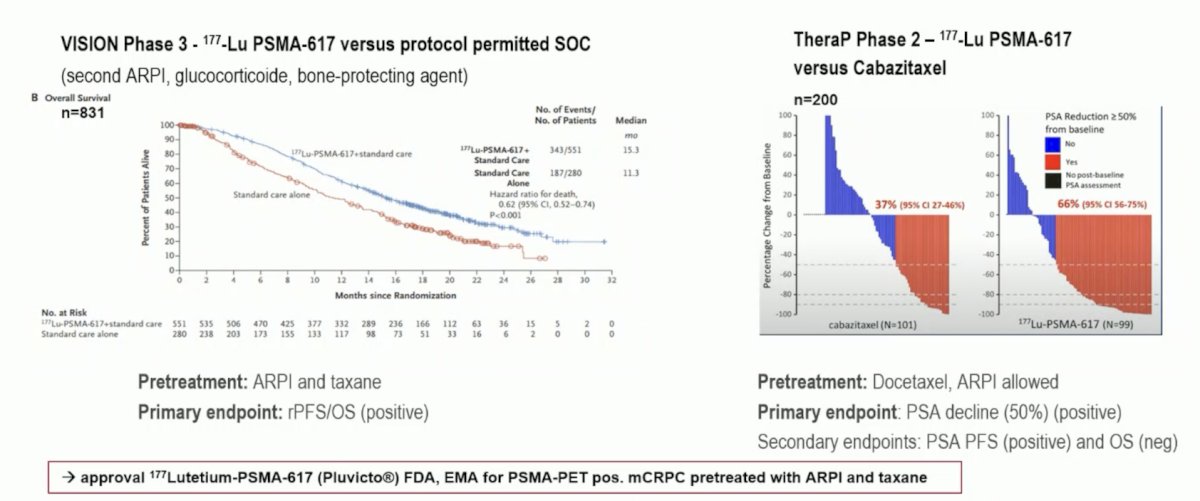
More recently, 177Lu-PSMA-617 has been assessed in the mCRPC setting post ARPI and taxane naïve in the PSMAfore trial,3 demonstrating a radiographic progression-free survival benefit, although overall survival data is immature. As of a December 2023 press release, the SPLASH trial, presented at ESMO 2024, stated there was a positive radiographic progression-free survival benefit of 9.5 versus 6.0 months (HR 0.71) with 177Lu-PNT2002. Dr. Vogl provided the following table highlighting the characteristics of the two phase 3 trials in mCRPC taxane naïve patients: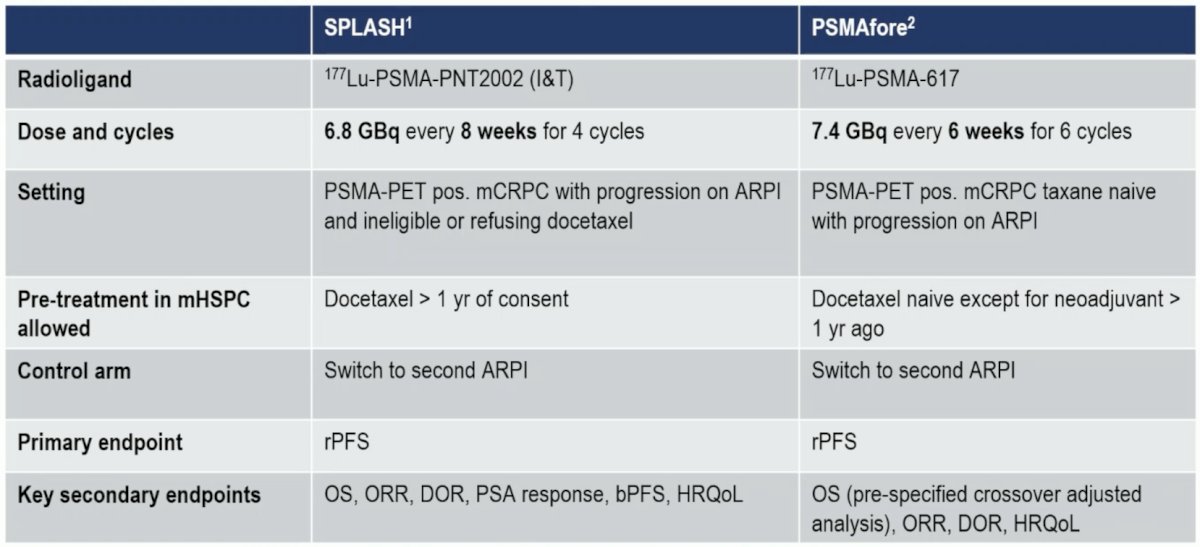
The median patient age was 72 years, and the median PSA was lower in the experimental arm (13.2 versus 19 ng/ml). Approximately 17% of patients had received prior taxanes for castrate-sensitive disease, and most patients (90%) had received the prior ARPI therapy in an M1 state. At the data cut-off date of November 1st, 2023, with a median follow-up of ~12 months, this trial met its primary endpoint of a radiographic progression-free survival benefit with 177Lu-PNT2002 (median: 9.5 versus 6 months; HR: 0.71, 95% CI: 0.55–0.92, p = 0.0088):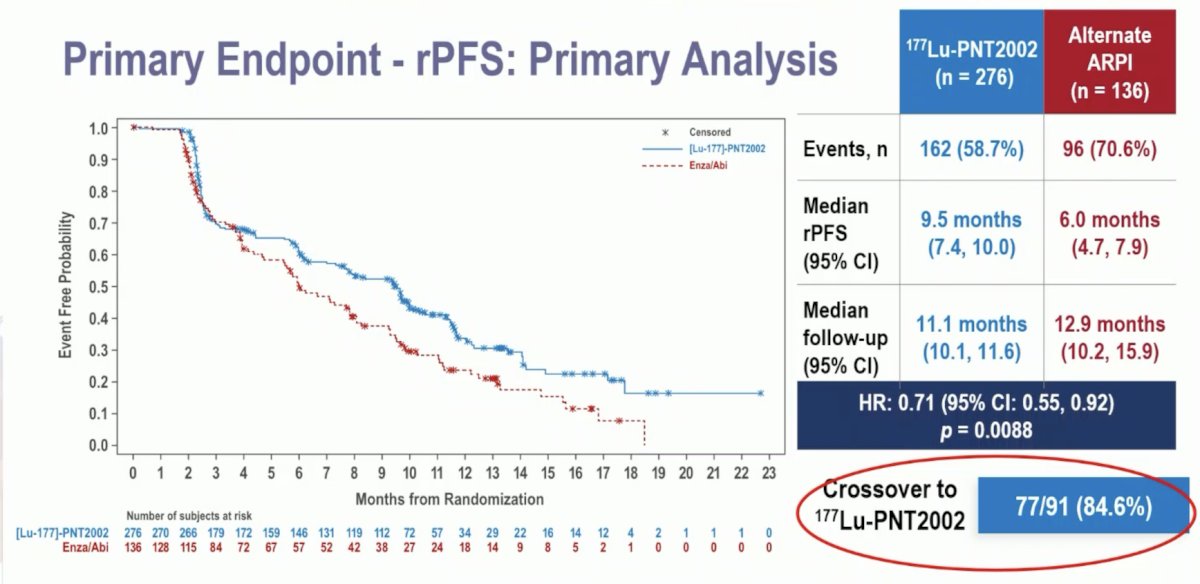
The first interim analysis of overall survival did not demonstrate an overall survival benefit for 177Lu-PNT2002 (median: 20.8 months versus non-estimable; HR: 1.11, 95% CI: 0.73–1.69, p = 0.62):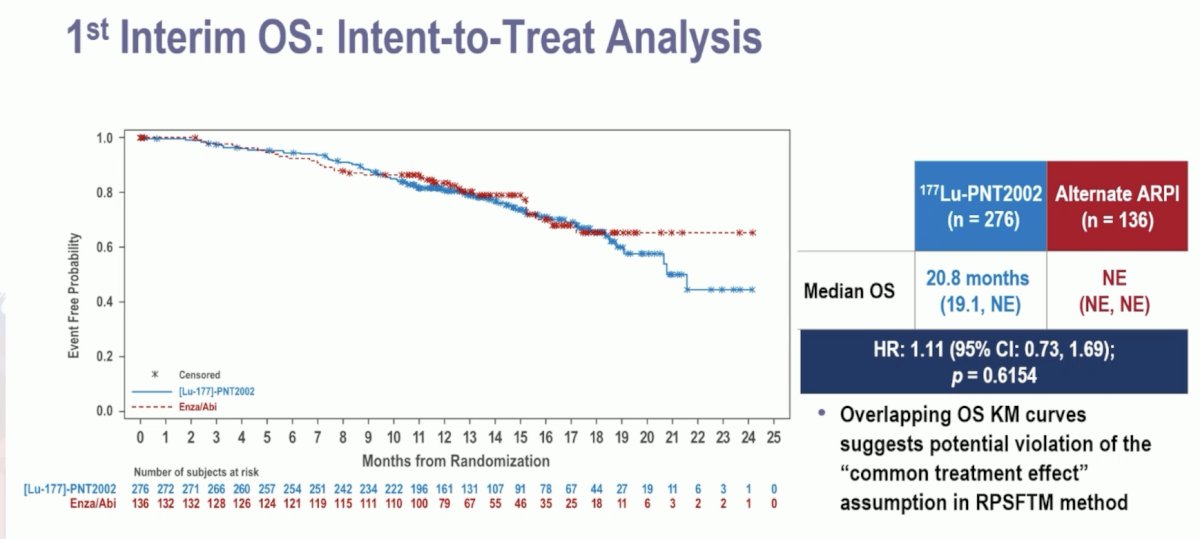
Any grade treatment-emergent adverse events were observed in nearly all patients. Overall, the adverse event profile favored 177Lu-PNT2002. Grade ≥3 events were observed in 10% and 12% of patients in the experimental and control arms, respectively. There were no treatment-related adverse events leading to death. Treatment-emergent adverse events leading to treatment discontinuation were observed in 2% and 6% of patients, respectively. Those leading to a reduction of study treatment were observed in 1% and 5%, respectively: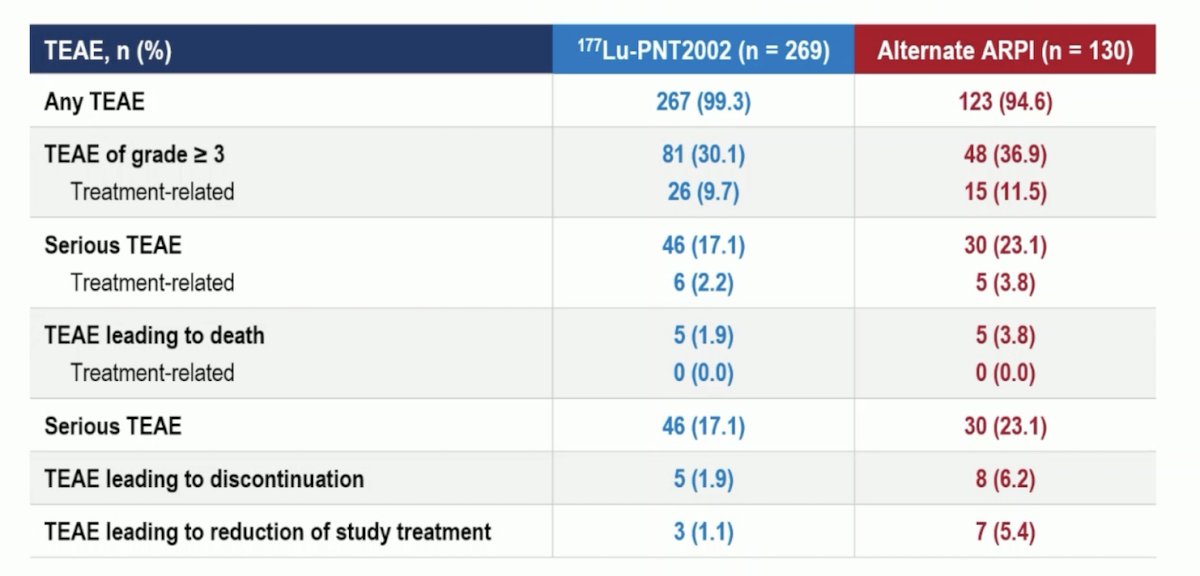
Grade ≥3 anemia was more common with 177Lu-PNT2002 (6% vs 3%). Arthralgia and fatigue were more common with the alternate ARPI: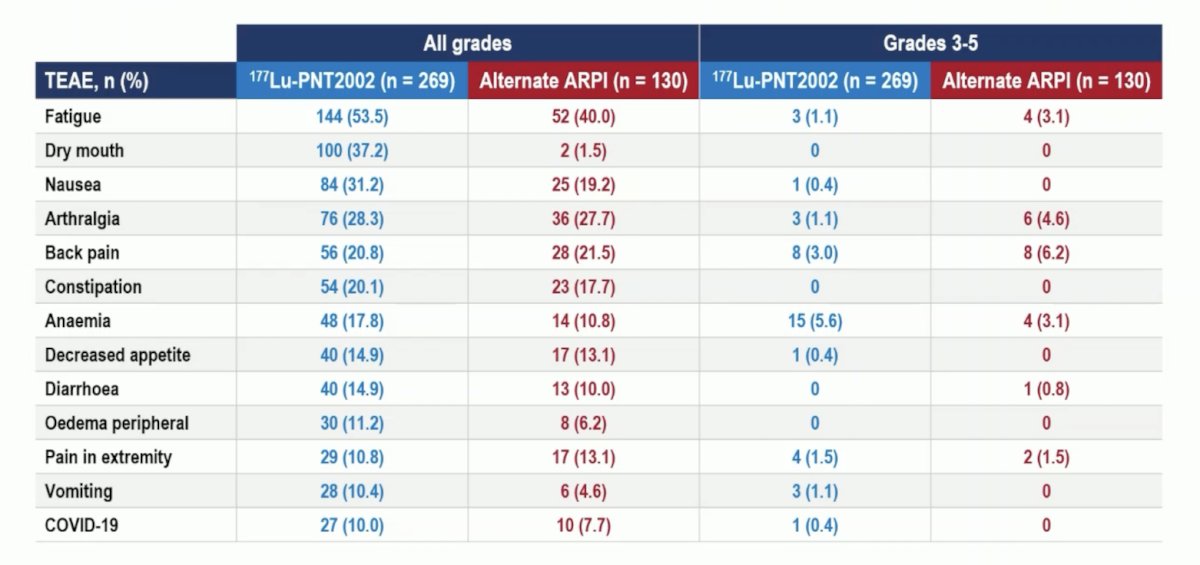
At the time of his presentation, Dr. Sartor’s conclusions for SPLASH included:
- 177Lu-PNT2002 reduced the risk of radiographic progression or death by 29% versus ARPI (HR 0.71, 95% CI 0.55-0.92)
- Overall survival data is immature
- 177Lu-PNT2002 (I&T) safely compared favorably to the ARPI control
SPLASH discussant Dr. Burger noted that optimal dosing and timing of Lu-PSMA therapy is still an open question.
Dr. Vogl concluded her presentation discussing highlights of the ESMO 2024 prostate cancer sessions with the following take-home points:
- There was excellent quality of research submitted to ESMO 2024
- There is not enough space to include all research that would have merited presentation
- ESMO 2024 was a fantastic meeting for prostate cancer researchers, clinicians taking home new treatment options, and providing inspiration for further research
Presented by: Ursula Vogl, MD, Professor, Oncology Institute of Southern Switzerland, Bellinzona, Switzerland
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: ESMO 2024: Scientific Congress Highlights: Genitourinary Tumors, Prostate
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Morris MJ, Castellano D, Herrmann K, et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naïve patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomized, controlled trial. Lancet 15 Sept 2024 [Epub ahead of print].


