(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a session focusing on rare genitourinary cancers. Dr. Angelika Terbuch discussed current controversies in testis cancer, focusing on the following topics:
- Clinical stage I seminoma: escalation of adjuvant treatment for selected groups?
- Metastatic, good prognosis seminoma: de-escalation or escalation?
- Stage II non-seminoma: Primary RPLND or chemotherapy?
The current standard of care for stage I seminoma is surveillance following a radical orchiectomy. However, in higher risk patients, 1 cycle of carboplatin may be offered.
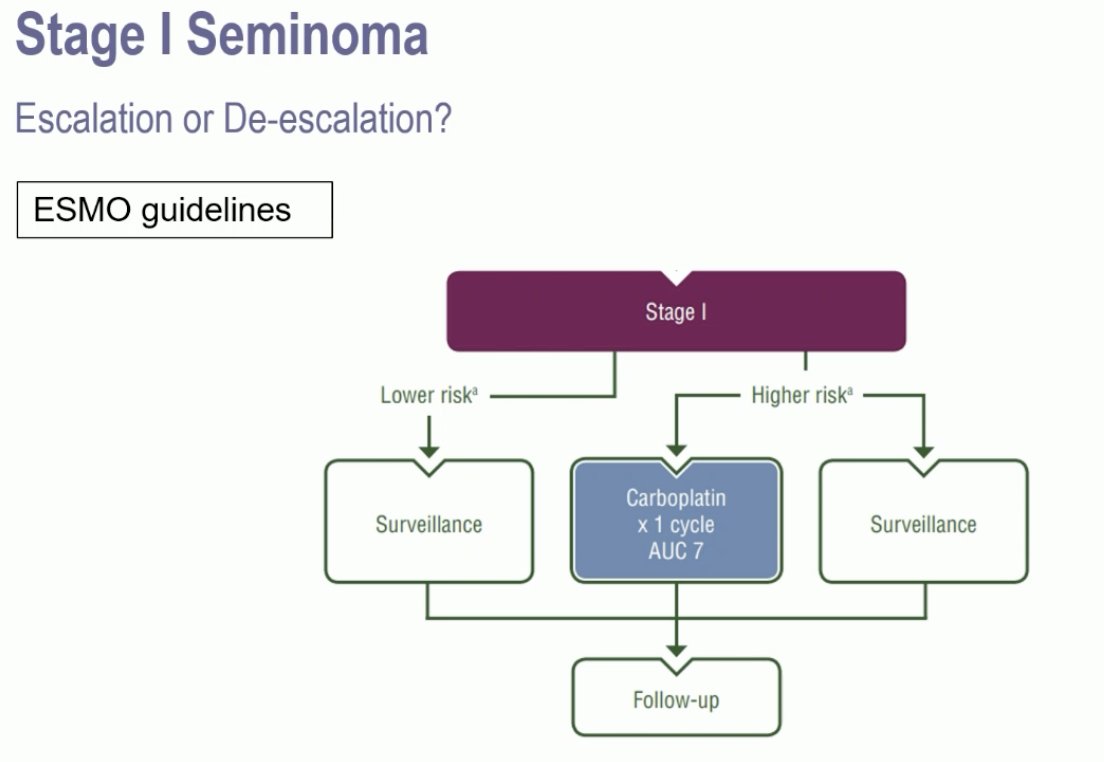
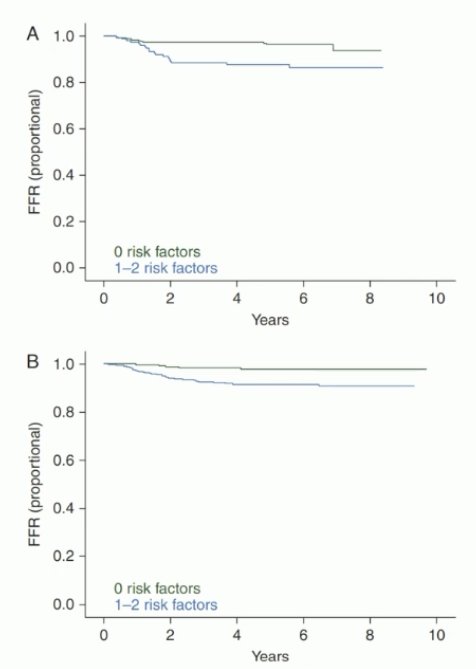
The risk factors for recurrence in these patients include rete testis invasion and tumor size >4 cm. In patients without risk factors who undergo active surveillance post-orchiectomy, the risk of recurrence is 4%. Following adjuvant carboplatin, this risk decreases to 2.2%. Conversely, in patients with 1–2 risk factors who undergo surveillance, the risk of recurrence is 15–20%. This is reduced to 9.3% if patients receive adjuvant carboplatin.1
Can we further improve on this 9.3% risk of recurrence? The SWENOTECA-ABC group is comparing adjuvant BEP (bleomycin, etoposide, and cisplatin) to carboplatin in stage I seminomatous testicular cancer patients. Dr. Terbuch noted that the recruitment goal has been achieved. But even if this trial is positive (i.e., it further reduces the risk of recurrence), it does not answer the question of who needs adjuvant therapy.
In 2024, the results of a Danish nationwide, population-based cohort study, published in The Journal of Clinical Oncology,2 demonstrated that prognostic factors for relapse in patients with clinical stage I testicular seminoma include:
- Infiltration of the testicular hilum, including the rete testis
- Lymphovascular invasion
- Elevated preoperative HCG levels
- Elevated preoperative LDH
The presence of all 4 risk factors was associated with a relapse risk of 62%.
Next, moving on to metastatic seminoma, Dr. Terbuch noted that treatment options for stage IIA patients include chemotherapy (BEP x 3 or EP x 4) or radiotherapy to the retroperitoneum. In higher stage tumors (IIB-III), chemotherapy (BEP x 3–4) is the recommended treatment.
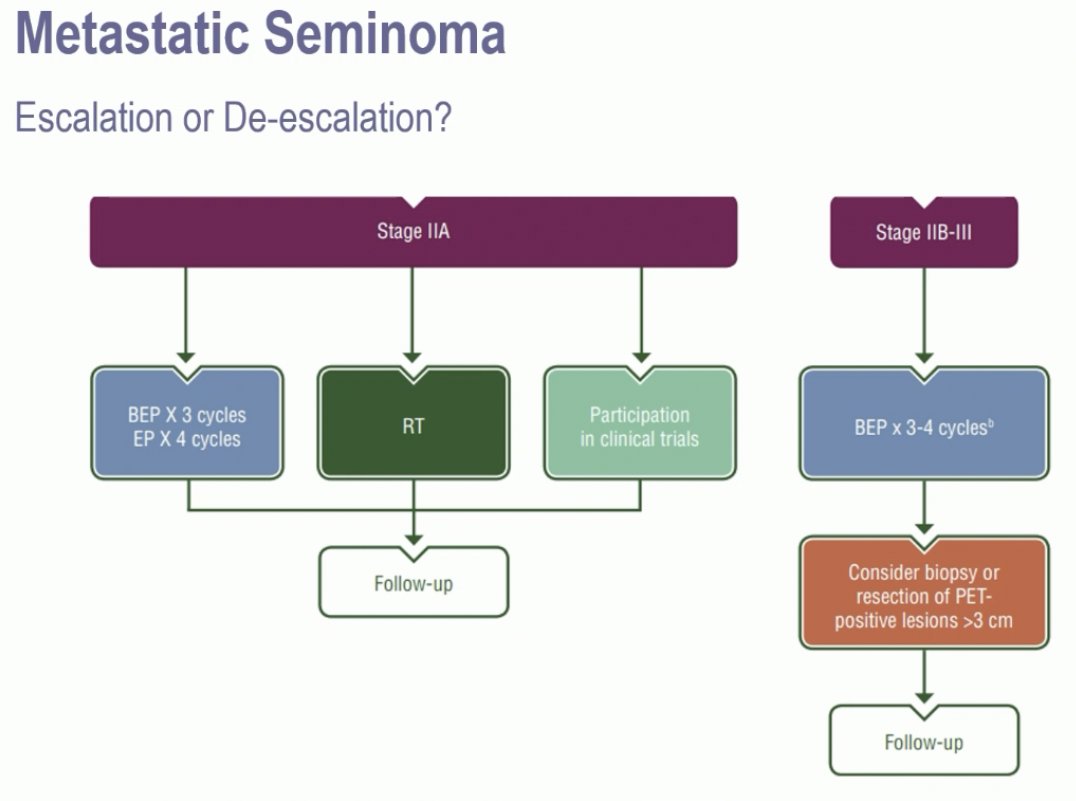
These chemotherapy regimens are associated with excellent survival outcomes. In the GETUG S99 trial, 4 cycles of EP was associated with 3-year progression-free and overall survivals of 93% and 99% in good prognosis seminoma patients. Conversely, in intermediate prognosis seminoma, the corresponding survival rates were 83% and 87%, respectively.3
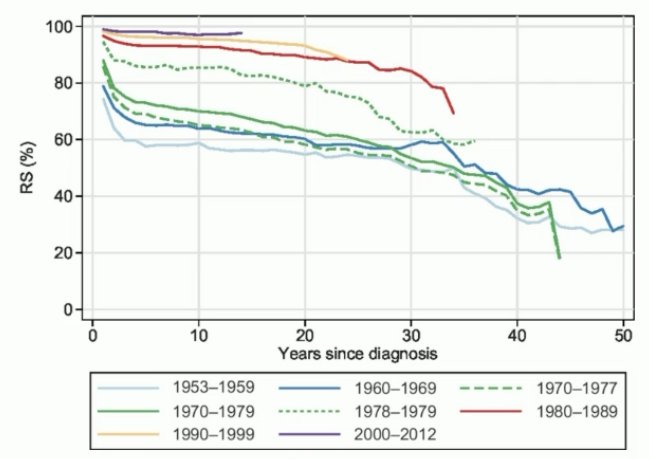
And while these cancer survival outcomes are impressive, it has long been established that chemotherapy is associated with long-term adverse effects, including cardiovascular toxicity and secondary malignancies, with an accelerated decline in long-term survival beyond 15–20 years.4
A de-escalation strategy has been assessed in the SEMITEP phase II trial in IGCCCG good prognosis seminoma patients. Patients received 2 cycles of EP, following which they underwent a PET scan. If the PET scan was negative, then patients received one additional cycle of carboplatin. If the PET was positive, then they received all 4 cycles of EP. Results of this trial were promising and demonstrated that the 3-year progression-free survival outcomes were similar in both groups of patients:5
- Carboplatin group: 90.8%
- EP group: 90%
SAKK 01/18 is an ongoing phase II trial evaluating single dose EP followed by involved-node only radiotherapy for stage IIA-B seminoma. PRIMETEST II is a phase II trial evaluating RPLND for stage IIA-B seminoma plus adjuvant chemotherapy for high-risk situations.
But not every IGCCCG good-risk patients is a candidate for de-escalation therapy. The IGCCCG classification is from 1997 and is based on data from 660 patients with metastatic seminoma. Additionally, good and intermediate prognostic groups were based on the presence of non-pulmonary visceral metastases.6 As such, there is heterogeneity within the prognostic groups. This is well-illustrated in the example below of two patients – both classified as good prognosis disease and recommended for the same treatment – with the 2nd patient having a significantly higher disease burden:
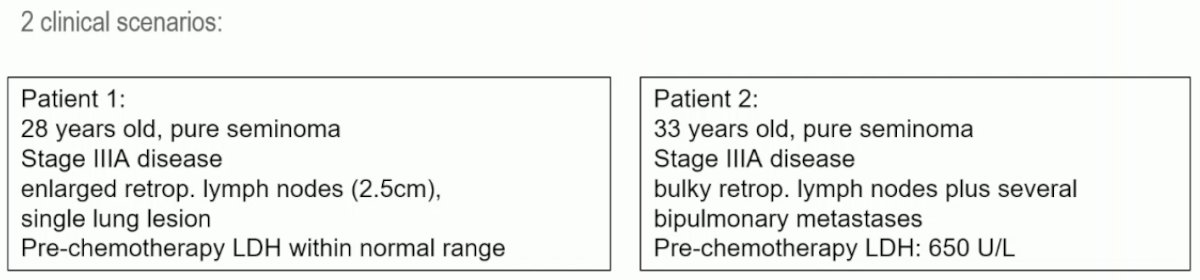
The IGCCCG classification was updated in 2021. This was based on data from 2,451 patients with metastatic seminoma. The original classification still identifies two prognostic groups. However, there is improved progression-free and overall survivals in modern series.7
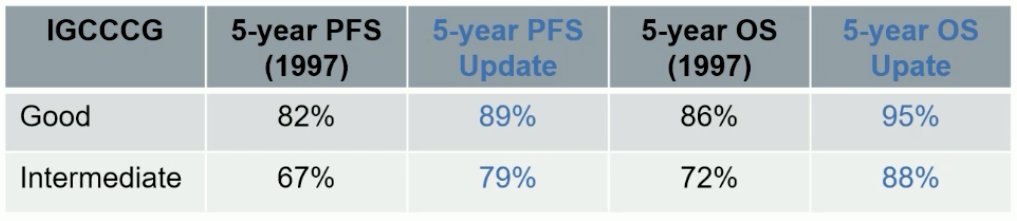
However, this update demonstrated that there are some patients in the good prognostic group that do not perform as well. Within the good prognosis group, LDH >2.5 was identified as an adverse prognostic factor. Their prognosis is similar to that of intermediate prognosis patients.
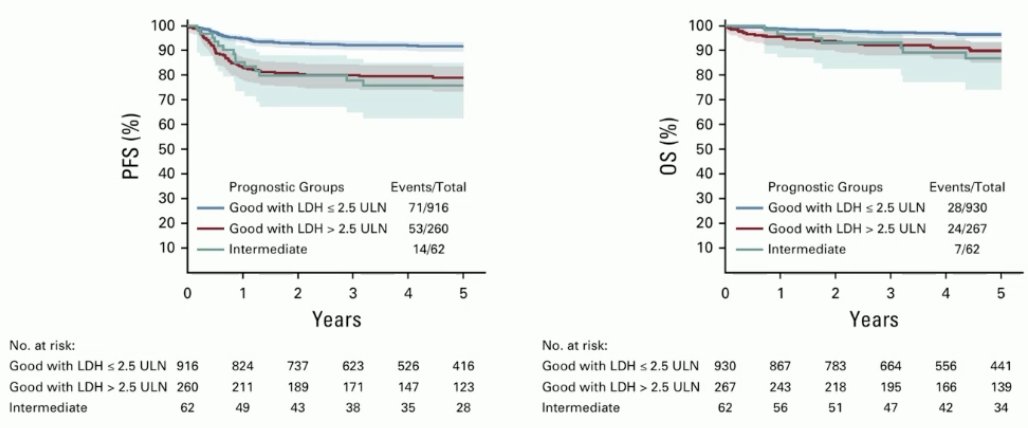
The new ESMO classification acknowledges the presence of heterogeneity within the good prognosis group, by dividing this group into lower and higher risk groups based on the LDH levels.
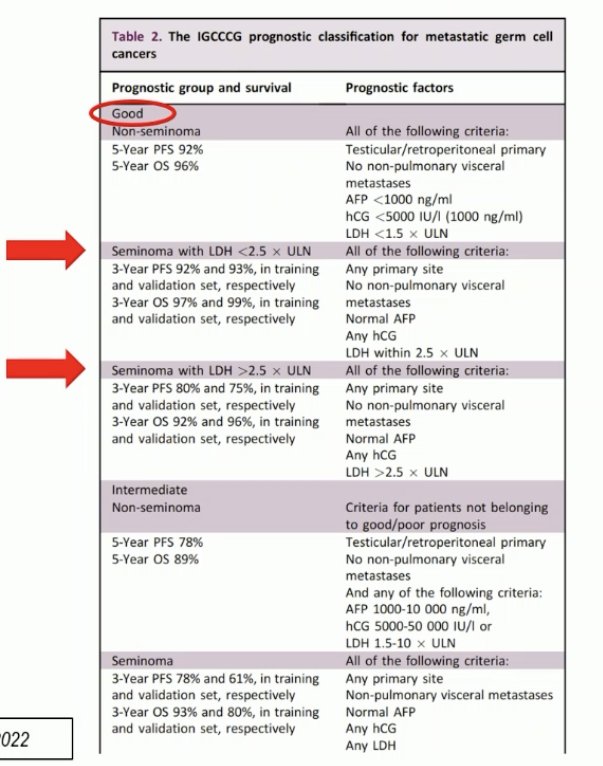
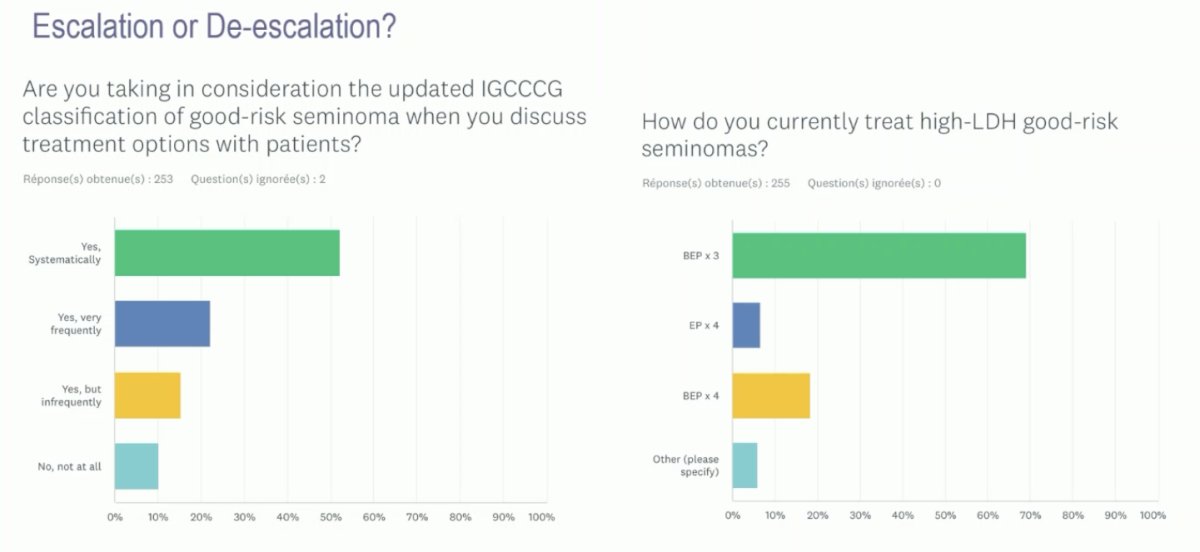
What is the contemporary use of the updated IGCCCG classification? >50% of clinicians noted that they use this updated classification on a systematic basis. From a treatment standpoint, the majority ~70% noted that BEP x 3 is their preferred treatment regimen to high LDH, good-risk seminomas.
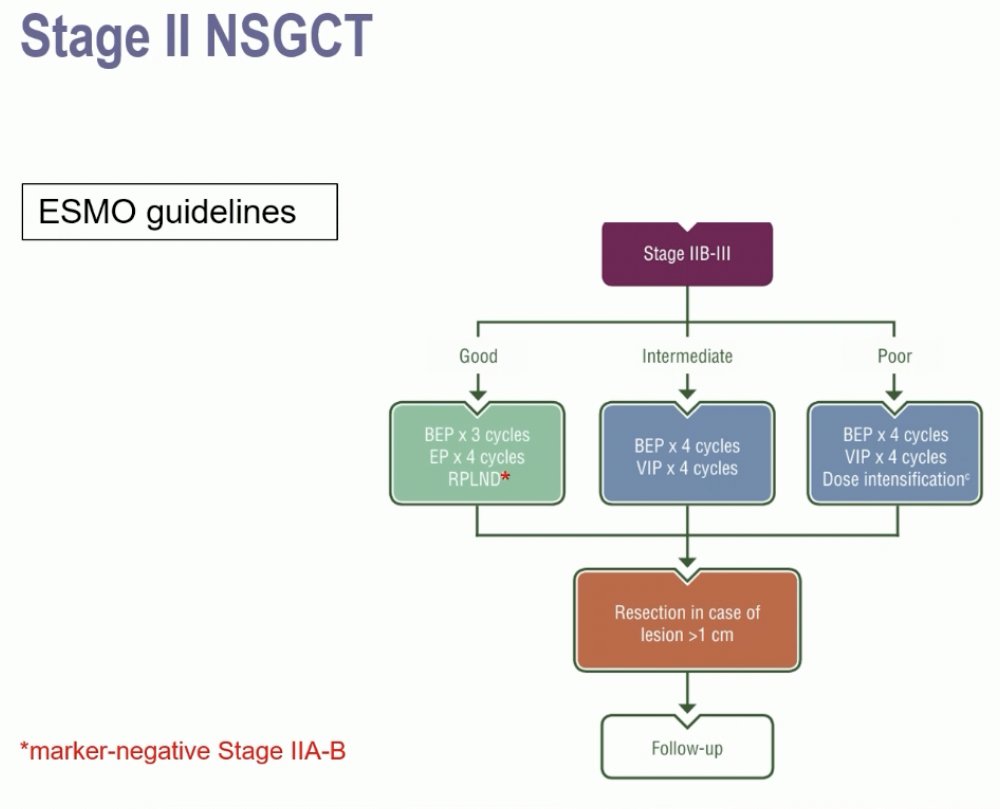
Next, moving on to discuss controversies in stage II non-seminomas, Dr. Terbuch noted that the current ESMO guidelines recommend chemotherapy for the majority of stage IIB-III NSGCTs, with RPLND an option for marker negative stage IIA-B patients.
One of the key points to emphasize is that 20–30% of stage IIA, marker-negative patients are actually pN0. As such, repeating a scan in 6 weeks is of significant value to differentiate the node positive from nod negative patients. In case of persistent marker negative stage IIA disease, an RPLND can be recommended. Dr. Terbuch highlighted the potential future role of miRNA 371a-3p in this setting.8,9
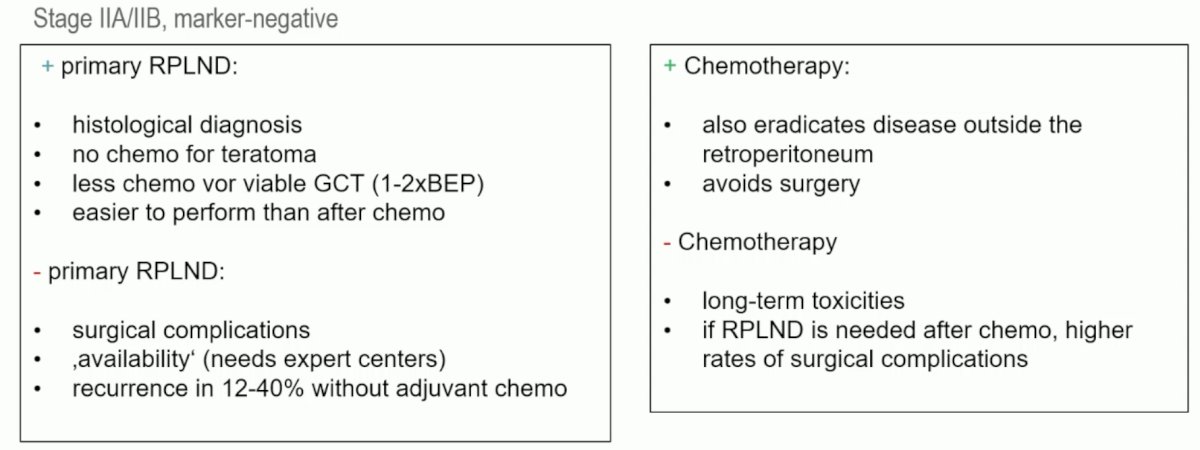
Summarized below are the advantages and disadvantages of each of primary RPLND and chemotherapy in this setting:
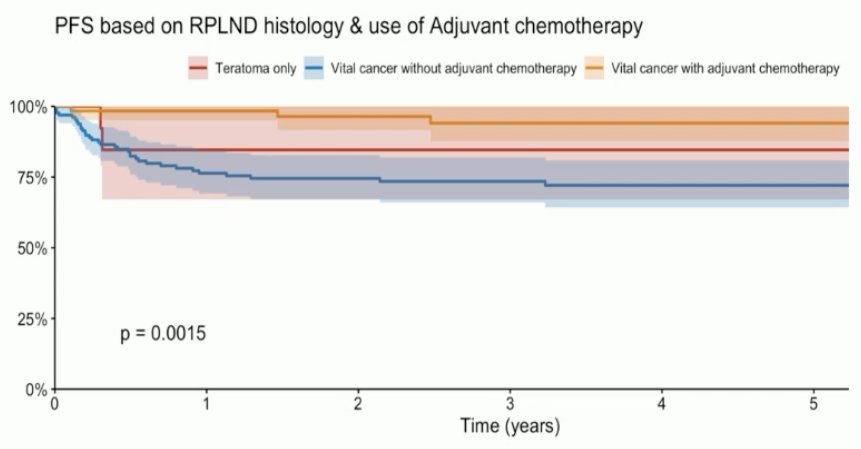
In unpublished data, the outcomes of 305 patients from 17 centers with stage IIA/B NSGCT (IIA: 70%) undergoing a primary RPLND were published. Among the 212 patients with clinical stage IIA disease, 33% had pN0 disease. This was also the case for 15% of patients with clinical stage IIB disease. Notably, pure teratoma was present in 6% of patients. Overall, these results suggest that 33% of patients would not have benefited from chemotherapy, based on pN0 status or presence of pure teratoma.
The 5-year progression-free survival rates were as follows:
- Viable cancer and adjuvant chemotherapy: 94%
- Pure teratoma: 85%
- Viable cancer without adjuvant chemotherapy: 72%
So, in summary, what are the selection criteria for primary RPLND?
- Tumor-marker negative disease (relapse rates up to 80% in marker positive disease, with high risk of systemic disease [HR: 5.6])
- Lymph nodes ≤3 cm
- Low number of enlarged lymph nodes
- Lymph nodes within the landing zone
In the current literature, the 5-year disease-free survival for these patients is ~70–80% without adjuvant chemotherapy. The best outcomes are for those with stage IIA disease (~90%). The 5-year disease-free survival is 93–99% with adjuvant chemotherapy.8,10-12
Dr. Terbuch concluded as follows:
- In clinical Stage I seminoma, the preferred management option is active surveillance, but with better selection criteria we might be able to identify patients who benefit from adjuvant treatment.
- In metastatic, good-prognosis seminoma patients with LDH levels < 2.5 x ULN, de-escalation strategies are possible.
- Metastatic, (good-prognosis) seminoma patients with LDH levels > 2.5 x ULN are not candidates for de-escalation strategies.
- In marker-negative clinical Stage IIA non-seminoma patients, initial surveillance followed by a second scan after 6 weeks should be performed.
Presented by: Angelika Terbuch, MD, Division of Oncology, Department of Internal Medicine, Medical University of Graz, Graz, Austria
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:
- Tandstad T, Stahl O, Dahl O, et al. Treatment of stage I seminoma, with one course of adjuvant carboplatin or surveillance, risk-adapted recommendations implementing patient autonomy: a report from the Swedish and Norwegian Testicular Cancer Group (SWENOTECA). Ann Oncol. 2016; 27(7):1299-304.
- Wagner T, Toft BG, Lauritsen J, et al. Prognostic Factors for Relapse in Patients With Clinical Stage I Testicular Seminoma: A Nationwide, Population-Based Cohort Study. J Clin Oncol. 2024; 42(1):81-9.
- Fizazi K, Delva R, Caty A, et al. A risk-adapted study of cisplatin and etoposide, with or without ifosfamide, in patients with metastatic seminoma: results of the GETUG S99 multicenter prospective study. Eur Urol. 2014; 65(2):381-6.
- Kvammen O, Myklebust TA, Solberg A, et al. Long-term Relative Survival after Diagnosis of Testicular Germ Cell Tumor. Cancer Epidemiol Biomarkers Prev. 2016; 25(5):773–9.
- Loriot Y, Texier M, Culine S, et al. The GETUG SEMITEP Trial: De-escalating Chemotherapy in Good-prognosis Seminoma Based on Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. Eur Urol. 2022; 82(2):172-9.
- Mead GM, Stenning SP. The International Germ Cell Consensus Classification: a new prognostic factor-based staging classification for metastatic germ cell tumours. Clin Oncol (R Coll Radiol). 1997; 9(4):207-9.
- Beyer J, Collette L, Sauve N, et al. Survival and New Prognosticators in Metastatic Seminoma: Results From the IGCCCG-Update Consortium. J Clin Oncol. 2021; 39(14):1553-62.
- Neuenschwander A, Lonati C, Antonelli L, et al. Treatment Outcomes for Men with Clinical Stage II Nonseminomatous Germ Cell Tumours Treated with Primary Retroperitoneal Lymph Node Dissection: A Systematic Review. Eur Urol Focus. 2023; 9(3):541-6.
- Lafin JT, Singla N, Woldu SL, et al. Serum MicroRNA-371a-3p Levels Predict Viable Germ Cell Tumor in Chemotherapy-naïve Patients Undergoing Retroperitoneal Lymph Node Dissection. Eur Urol. 2020; 77(2):290-2.
- Stephenson AJ, Bosl GJ, Motzer RJ, et al. Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: impact of patient selection factors on outcome. J Clin Oncol. 2005; 23(12):2781-8.
- Stephenson AJ, Bosl G, Motzer RJ, et al. Nonrandomized comparison of primary chemotherapy and retroperitoneal lymph node dissection for clinical stage IIA and IIB nonseminomatous germ cell testicular cancer. J Clin Oncol. 2007; 25(35):5597-602.
- Nicolai N, Nazzani S, Tesone A, et al. Retroperitoneal lymph-node dissection (RPLND) as upfront management in stage II germ-cell tumours: Evaluation of safety and efficacy. Tumori. 2023; 109(4):379-86.


