Dr. De Santis notes that there is poor data with low levels of evidence for systemic therapy options for patients with positive pelvic lymph nodes. As such, we must rely on data from subgroups of larger trials, namely STAMPEDE and GETUG-12. Importantly, Dr. De Santis notes that neither CHAARTED nor LATITUDE included patients that were exclusively N+. STAMPEDE inclusion criteria included men with locally advanced or metastatic prostate cancer, including newly diagnosed with N1 or M1 disease, or any two of the following: stage T3/4, PSA ≥ 40 ng/mL, or Gleason score 8-10. Patients undergoing prior radical prostatectomy or RT were eligible if they had more than one of the following: PSA ≥ 4 ng/mL and PSADT < 6 months, PSA ≥ 20 ng/mL, N1, or M1 disease. Looking closer at the STAMPEDE abiraterone study1, there were 182 and 187 patients in the treatment and control arm, respectively, who were newly diagnosed N1M0. In a preplanned analysis based on metastatic status, the M0 (N+) data had 78 deaths and was thus immature at the time of initial analysis to detect a survival difference between patients receiving abiraterone + prednisone + ADT vs ADT only (HR 0.75, 95%CI 0.48-1.18). However, in the preplanned analysis of failure-free survival, patients with M0 (N+) disease treated with abiraterone + prednisone + ADT had a significant advantage (HR 0.21, 95%CI 0.15-0.31).
At the 2018 ESMO meeting, an update was presented for the GETUG-12 trial2, a phase 3 trial of docetaxel-based chemotherapy in high-risk localized prostate cancer. The trial design of GETUG-12 is as follows:

Between 2002-2006 there were 413 patients recruited; among these patients were pN+, which comprised 29% of each trial arm. The primary endpoint of this trial was relapsed free-survival, which favored docetaxel + estramustine + ADT (HR 0.71, 95%CI 0.55-0.93). In a subgroup analysis of pN=1 or 2 patients, there was also an RFS benefit with docetaxel + estramustine + ADT (HR 0.64, 95%CI 0.43-0.95). Dr. De Santis poses the question, is this practice changing? Unlikely. In Dr. De Santis’ opinion, there are two trials planned/ongoing that encompass the N+ disease space that will likely be practice changing.
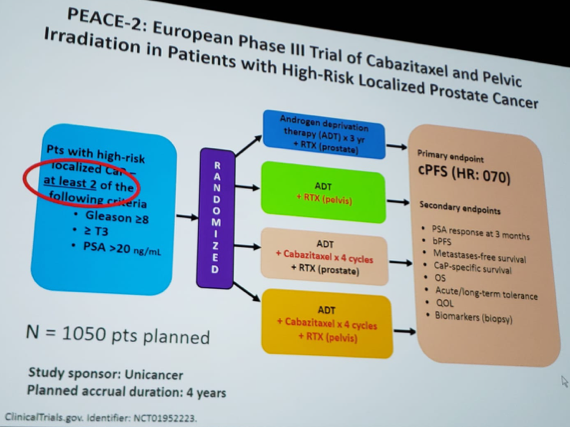
PEACE-2:
ENZARAD:

Dr. De Santis concluded her presentation with several take-home points:
- Docetaxel or abiraterone added to ADT are experimental for localized and N+ prostate cancer
- There is evolving evidence that in M0HSPC and “highest-risk” localized prostate cancer additional systemic therapy (chemotherapy or novel hormones) is improving outcomes
- There are ongoing trials for high-risk localized disease exploring multimodal therapy including systemic treatment
Presented by: Maria De Santis, medical oncologist and Chair of Section for Interdisciplinary Genito-Urinary Cancer Medicine at the Charité Medical University Hospital, Berlin, Germany.
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md, at the 16th Meeting of the European Section of Oncological Urology, #ESOU19, January 18-20, 2019, Prague, Czech Republic
References:
1. James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
2. Fizazi K, Carmel A, Joly F, et al. Updated results of GETUG-12, a phase 3 trial of docetaxel-based chemotherapy in high-risk localized prostate cancer with 12-year follow-up. Ann Oncol 2018;29(suppl_8): Abstract 791O.
Further Related Content:
Treatment of Positive Pelvic Lymph Nodes: Surgery
Treatment of Positive Pelvic Lymph Nodes: Genomics
Treatment of Positive Pelvic Lymph Nodes: Radio Hormonal Therapy


