Molecular Classification of Urothelial Carcinoma
More than most of the other GU malignancies, the genomic landscape and understanding of bladder cancer have been rapidly growing. Since the TCGA paper in 2014, there have been numerous groups that have come forward with relatively similar molecular classifications of MIBC. Kamoun A. et al.1 (in an as-of-yet unpublished paper) provide a consensus classification system that brings together all the current data. The data is summarized in the slide below:
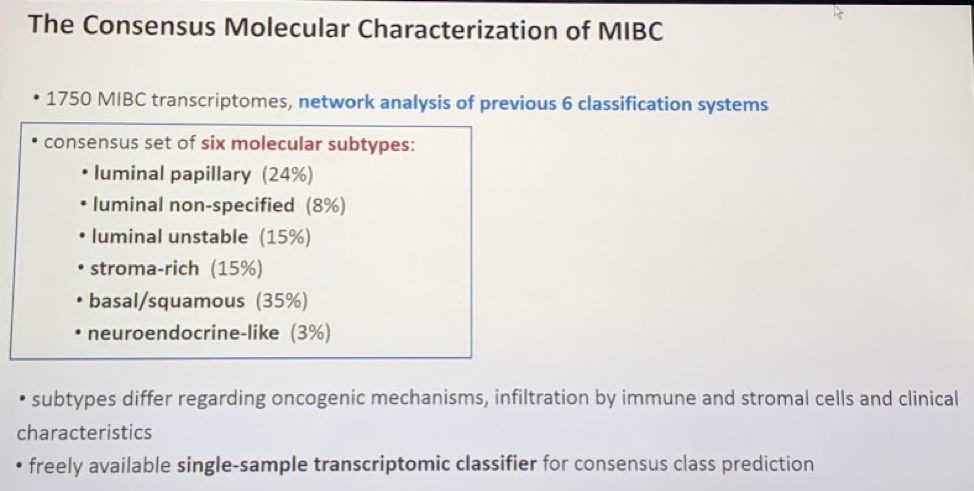
More importantly, each of these 6 molecular subtypes, based primarily on retrospective data, are associated with variable responses to neoadjuvant chemotherapy and alternative systemic therapies, such as immunotherapy. They will therefore play an important role in future bladder cancer treatment paradigms. The framework for testing and validating these as biomarkers are already underway.
NMIBC (non-muscle-invasive bladder cancer)
- van de Putte et al.2 – in this study, the authors assess two commonly used (albeit infrequently) substaging systems for cT1 NMIBC. The first is the metric substaging system into cT1m (microinvasive, < 0.5 mm lamina propria invasion) and cT1e (extensive, > 0.5 mm). The second is the cT1a/b substaging into cT1a (invasion above or into muscularis mucosae) and cT1b (invasion beyond muscular mucosae). They assessed 601 cT1 bladder tumors treated with TURBT and BCG for prognostic value to predict PFS and CSS. They found that, with median follow-up 5.9 years, there was clear benefit to using the metric system (better separation in PFS KM curves) and better interobserver concordance.
- de Wit et al.3 and the Keynote-057 study – albeit just a presentation so far and not yet published, this study provided key early information suggesting a role for immune checkpoint inhibitors in the NMIBC setting. Specifically for high-risk BCG unresponsive NMIBC. With median follow-up 9+ months, they found that 36.5% of patients had CR at 3 months. Of these patients, >80% had CR duration >= 6 months. It was also well tolerated.
- Gschwend et al.4 – in this prospective, randomized trial of 401 patients between 2006-2010 who underwent radical cystectomy, patients were randomized to extended or limited PLND. The primary outcome was recurrence-free survival. There was no clinically significant difference in RFS at 5-years: 65% vs. 59% (HR 0.84, p = 0.36). They removed 31 nodes (median) in the ePLND and 19 (median) in the lPLND. However, the population was enriched with cT1 and cT2 patients, so may not have been the right population in hindsight. SWOG 1011 is due to report in 2022 and may shed more light on this.
- Necchi et al.5 and the PURE-01 trial – in this excellent phase II open-label study, the authors are the first to assess the utility of immune checkpoint inhibitors in the neoadjuvant setting. 47 patients received 3 cycles of pembrolizumab, with 12 weeks between last dose and radical cystectomy. The drug was tolerated well. In the overall population, pathologic CR was seen in 42% of patients and pathologic downstaging in 54% - of note, in patients who were PD-L1+ (>10%), rates were higher at 54% and 65% respectively. There was also an association with tumor mutational burden and pT0 response. Hence, this may be considered a good option for cisplatin-ineligible patients at this time. However, further phase III studies are needed.
- Powles et al.6 and IMVIGOR211 – in this multicenter open-label phase III randomized control trial, atezolizumab was compared head-to-head against current 2nd line systemic therapy (vinflunine or taxanes) in platinum-refractory metastatic urothelial carcinoma. Randomized stratified based on PD-L1 status and the primary endpoint was OS. They found that there was no difference in overall survival in the IC2/3 population. High PD-L1 was associated with improved outcomes with both chemo and atezo. In contrast, higher tumor mutational burden predicted OS in favor of atezo; however, clinical benefit with atezo was also seen in the ITT population. There was less toxicity with atezo (20% vs. 43%). Hence immune checkpoint inhibitors are the preferred second-line therapy for metastatic bladder cancer that has progressed on cisplatinum.
- Birtle et al.7 and the POUT study – this study has previously been presented by Dr. Jones at this meeting, so he didn’t dwell on it too much. Ultimately, this study demonstrated that adjuvant platinum therapy improved DFS and MFS in patients with UTUC following radical nephroureterectomy. This will likely be the new standard of care.
Presented by: Michal Stanik, Masaryk Memorial Cancer Institute, Department of Urologic Oncology, Brno, Czech Republic
Written by: Thenappan Chandrasekar, MD. Clinical Instructor, Thomas Jefferson University, Twitter: @tchandra_uromd, @TjuUrology, at the 16th Meeting of the European Section of Oncological Urology, #ESOU19, January 18-20, 2019, Prague, Czech Republic
References:
1. Aurelie Kamoun et al. The consensus molecular classification of muscle-invasive bladder cancer. Pre-print at https://www.biorxiv.org/content/early/2018/12/10/488460.full.pdf+html.
2. Fransen van de Putte EE, et al. Metric substage according to micro and extensive lamina propria invasion improves prognostics in T1 bladder cancer. Urol Oncol. 2018 Aug;36(8):361.e7-361.e13. doi: 10.1016/j.urolonc.2018.05.007. Epub 2018 Jun 5.
3. de Wit et al. Pembrolizumab for High-Risk Non–Muscle Invasive Bladder Cancer (NMIBC) Unresponsive to BCG: Phase 2 Keynote-057 Trial. ESMO 2018.
4. Gschwend JE, et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol. 2018 Oct 15. pii: S0302-2838(18)30737-1. doi: 10.1016/j.eururo.2018.09.047. [Epub ahead of print]
5. Necchi A, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol. 2018 Oct 20:JCO1801148. doi: 10.1200/JCO.18.01148. [Epub ahead of print]
6. Powles T, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018 Feb 24;391(10122):748-757. doi: 10.1016/S0140-6736(17)33297-X. Epub 2017 Dec 18.
7. Birtle A et al. “POUT: A Phase III Randomized Trial of Perioperative Chemotherapy versus Surveillance in Upper Tract Urothelial Carcinoma.” ASCO GU 2018.
Further Related Content:
Best of Uro-Oncology 2018: Renal Cancer
Best of Uro-Oncology 2018 - Prostate Cancer
Best of Uro-Oncology 2018: Penile and Testis Cancer


