At this time, there are 4 reported in the adjuvant setting - ASSURE (Haas NB et al. Lancet 2016), S-TRAC (Ravaud A et al. NEJM 2016), PROTECT trials (Motzer RJ et al. JCO 2017), and ATLAS (Gross-Goupil M et al. Ann Oncol 2018). There are 2 ongoing trials that are likely to report soon – SORCE and EVEREST.
He started off by reviewing his criteria for effective adjuvant therapy – and based the rest of his talk on whether the current therapeutic options met those criteria. Adjuvant therapy is based on effective systemic therapy for known metastatic disease – so those agents must be effective in the metastatic setting. Once that is established, they must then have a good “cost”/benefit ratio – specifically, they must have a high-risk population that would benefit from treatment, have a relevant endpoint that is met, and their toxicity profile must be tolerable.
There is no doubt that the tested agents – sunitinib, sorafenib, pazopanib, axitinib – are established agents for the treatment of metastatic RCC. They make up the first and second line for mRCC and there is strong RCT data to support their use. So, these agents meet the first criteria for adjuvant therapy.
However, how do they fare from a “cost”/benefit ratio?
1) High-risk population
He provided a very nice hand-made slide summarizing the specific population tested in each of the 4 RCTs. S-TRAC was the most selective for high-risk patients, but the others include some intermediate risk patients.
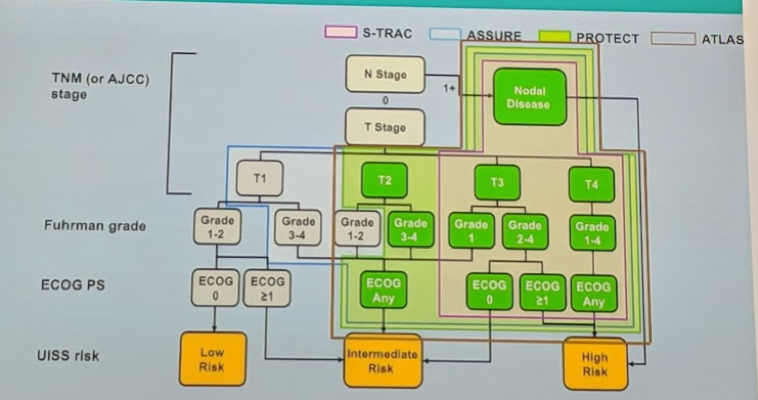
While different risk models can be used (UISS modified or SSIGN), these are relatively similar in risk stratification. So, based on the above, the studies are mixed with regards to high-risk patient selection.
2) Relevant Endpoint met
The endpoint for all the studies was disease-free survival (DFS), rather than the more important overall survival (OS). He briefly reviewed the outcomes of the 4 studies – again emphasizing that only S-TRAC met DFS endpoint (6% 3 and 5-year DFS absolute benefit, HR 0.76, p = 0.03).
A recent study by Harshman et al (Cancer 2017) demonstrated that on a systematic review of 13 RCTs encompassing 6473 patients, there was only a modest correlation between 5-year DFS and 5-year OS. So perhaps this isn’t a relevant endpoint for evaluation.
3) Toxicity – all the 4 RCTs studies suffered from significant rates of drug discontinuation due to toxicity (23-45%), much higher than discontinuation rates seen in the metastatic RCC population. This nice table summarizes this:

So, likely due to the absence of clinically evident disease and no symptoms from the disease itself, patients do not tolerate these agents in an adjuvant setting.
Based on these medications not meeting the “cost”/benefit ratio, he also recommends against the use of adjuvant therapy in high-risk RCC management.
His main conclusions were:

Presented by: Aristotelis Bamias, Αssociate Professor at the Medical School, University of Athens, Athens, Greece
Written by: Thenappan Chandrasekar, MD. Clinical Instructor, Thomas Jefferson University, Twitter: @tchandra_uromd, @TjuUrology, at the 16th Meeting of the European Section of Oncological Urology, #ESOU19, January 18-20, 2019, Prague, Czech Republic
Further Related Content:
Adjuvant Therapy after Nephrectomy for High-Risk Renal Cell Carcinoma: The Urologist's Perspective


