He began by highlighting the EORTC GU 30012 (Gore ME et al. Lancet 2010) study assessing IFN-alpha vs. IFN-alpha/IL-2/fluorouracil in the treatment of mRCC, the largest RCT assessing aspecific immunotherapy (first general immunotherapy in RCC). In that study, there was no disease-free (DFS) or overall survival (OS) benefit, but they did note the following: these agents generated a class effect of fever-like side effects.
Around that same time, increased knowledge and understanding of the pathogenesis of RCC led to the identification of angiogenesis pathways, such as VEGF, mTOR, etc., that led to the development of the targeted therapies. This led to agents that targeted these pathways and was found to have important clinical effects (usually not cure, but rather control) on mRCC patients.
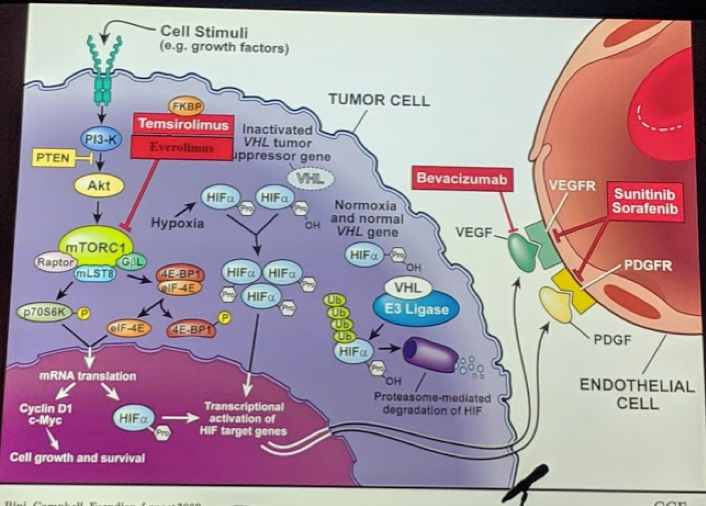
The first of the studies was the Sunitinib study (Motzer et al. NEJM 2007) led to it being the first established agent with potent OS benefit in mRCC. But, as these agents developed, these too appeared to have a class-effect of an adverse event profile, including diarrhea, nausea, hand-foot syndrome, fatigue, etc. Eventually, the COMPARZ study brought pazopanib to the front line as well – as it had slightly better toxicity profile and similar oncologic efficacy.
Then, in 2013, the next generation of immunotherapy began to develop. They built on the prior work of specific immunotherapy, and helped patients’ immune systems overcome the tumor evasion techniques. In doing so, they ramped up T-cell activity and caused tumor cell death.

This naturally led to the development of clinical agents that could target RCC.
- CheckMate-214 (Motzer et al. NEJM 2018): RCT randomized patients 1:1 Nivolumab/Ipililumubab vs. Sutent. Found that in intermediate/poor risk patients, it outperformed sunitinib with regards to objective response rate (ORR), PFS, and OS.
- Similar to prior studies, the immune checkpoint inhibitors had a class effect in terms of adverse event profile
- Generally well tolerated (better than targeted therapies), but rare deaths did occur due to immune-mediated toxicity
- InMotion 150 (phase II trial, Atkins MB et al) – RCT randomizing patients 1:1:1 to atezolizumab vs. atezo/bevacizuab vs. sunitinib. In this study, the authors identified encouraging PFS benefit in patients treated with both atezo/bev compared to atezo alone and sunitinib alone, but only in the patients with PD-L1 expression > 5%. Based on this study, this was taken to a phase III trial (InMotion 151)
- InMotion 151 (phase III trial, Motzer et al. GU ASCO 2018) – RCT comparing atezo/bev vs. sunitinib alone. They identified a PFS benefit in patients with PD-L1+ expression > 1% (HR 0.74, p = 0.02).
- JAVELIN Renal 101 (Motzer et al. ESMO 2018) – first RCT combining an immune checkpoint inhibitor with a TKI. They compared avelumab / axitinib vs sunitinib alone. They identified a significant PFS benefit (HR 0.61, p < 0.001) in patients with PD-L1+ tumors. OS benefit in the entire cohort was not statistically significant, but the date is still immature.
- Subgroup analysis appears to favor the combination over sunitinib in all groups – though some were underpowered to identify a significant improvement
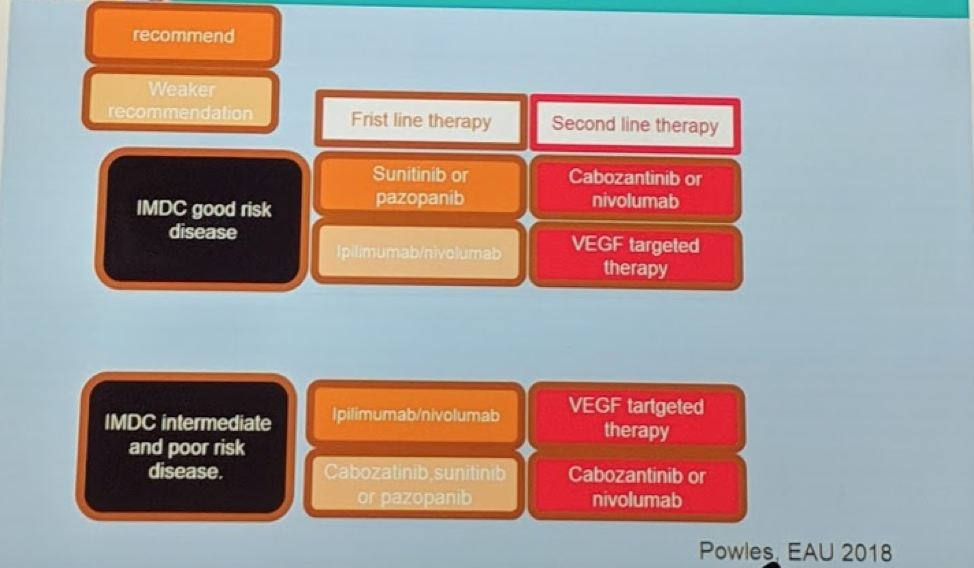
Based on all of this data, the current paradigm for mRCC treatment is as follows (adapted from T. Powles’ presentation at EAU 2018):
Presented by: Peter Mulders, MD, Professor, Chair of Urology, Radboud University Medical Centre, Nijmegen, the Netherlands
Written by: Thenappan Chandrasekar, MD. Clinical Instructor, Thomas Jefferson University, Twitter: @tchandra_uromd, @TjuUrology, at the 16th Meeting of the European Section of Oncological Urology, #ESOU19, January 18-20, 2019, Prague, Czech Republic


