Torgrim Tandstad, MD, PhD, notes that the goals for CS I and CS II patients is to prevent over or under treatment, as there is a possibility that small lymph nodes comprising CS IIA patients are benign. As part of the SWENOTECA initiative, Dr. Tandstad states that their algorithm for all CS I and CS IIA patients is delayed staging. This encompasses the following steps after radical orchiectomy: (i) weekly measurement of tumor markers if primarily elevated, and (ii) new diagnostic imaging 6-8 weeks after orchiectomy. According to Dr. Tandstad, this gives much more accurate staging without any risk to the patient and allows assurance of metastatic disease before treatment.
For patients with CS IIA/B tumors, cure rate is not an endpoint, but a quality indicator. The burden of treatment (short term effects) and long-term morbidity/survival (late effects) are the primary endpoints for these patients. Relapses are a burden for the patient and physician and should be as low as possible. Dr. Tandstad notes that the rationale for primary RPLND in CS IIA/B patients is it reduces the need for chemotherapy and the late toxicity associated with secondary malignancies, as well as some patients not having vital germ cell cancer (ie. teratoma or benign lymph nodes).
The SWENOTECA group uses the following algorithm for CS IIA marker negative disease, started in 2011 (SWENOTECA VIII):
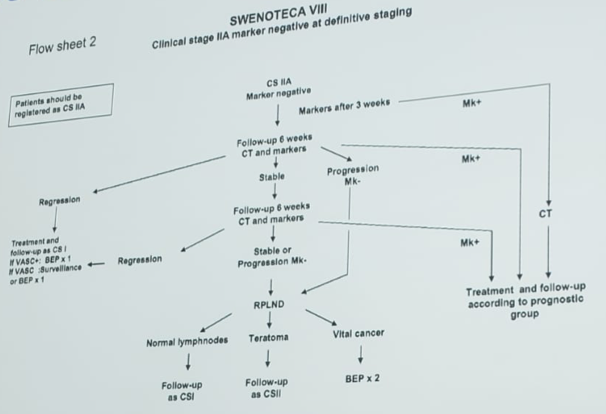
Using this algorithm, 20 patients had an RPLND (6 benign, 13 vital GCT, 1 vital GCT + teratoma; 10% complication rate) among which all patients with vital GCT received 2 cycles of adjuvant BEP. There were no relapses and 95% OS rate (one unrelated death) over a median 56 months. In another 57 patients, all were treated with BEP x3 of which 15% eventually needed an RPLND. The OS rate was 100% and PFS rate was 95%.
Dr. Tandstad then highlighted his differences between BEP x3 vs RPLND among patients with NSGCT CS IIA/B disease:
- BEP x3: is safe but may have complications, is a standardized treatment, has unprecedented OS and RFS in a population-based setting, and 15% of patients will require chemotherapy + RPLND
- RPLND: is safe but may have complications, is highly dependent on the surgeon, and most patients will need chemotherapy regardless or have a high risk of relapse
- BEP x3: is a safe treatment but with known complications, is a standardized treatment, and has unprecedented OS and RFS in a population-based setting
- RPLND: is a safe treatment but with known complications, is highly dependent on the surgeon, has not evaluated in a population-based setting, leads to more relapses, and is still experimental and should only be performed in prospective protocols
- Never treat CS IIA unless metastatic disease is unequivocal
- Based on the current knowledge, BEP x3 should be the standard treatment of good prognosis testicular cancer regardless of stage and markers
- RPLND should preferably be evaluated prospectively in larger population-based studies before it accurately can be evaluated as a standard therapy against chemotherapy
Read the Opposing Side of the Debate: The Role of RPLND in Tumor Marker Negative Stage IIA-B: PRO
Written By: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 16th Meeting of the European Section of Oncological Urology, #ESOU19, January 18-20, 2019, Prague, Czech Republic


