Dr. Seisen discussed the DaBlaCa-13 study, which was designed to assess tumor response and adverse events related to short-term, intensive chemoresection with mitomycin C compared with adjuvant instillations in patients with recurrent non-muscle-invasive bladder cancer (NMIBC).1 Patients were randomized to intravesical mitomycin C three times a week for 2 weeks in the intervention group (59 patients) versus transurethral removal of bladder tumor (TURBT) and six weekly adjuvant instillations in the control group (61 patients). The primary outcome was tumor response and was evaluated in the intervention group by flexible cystoscopy after 4 weeks of treatment. The DaBlaCa-13 trial found that complete tumor response was seen in 33 participants (57%) in the intervention group, and fewer adverse events were reported in the intervention group than in the control group. Dr. Seisen noted that these results are provocative given that chemoresection yielded a tumor response in 57% of patients, and thus only half of the patients treated with chemoresection truly needed a TURBT.
The Phase III NIMBUS trial was also published in European Urology in 2020, assessing treatment of high-grade NMIBC by standard number and dose of Bacillus Calmette-Guérin (BCG) instillations compared to a reduced number and standard dose of BCG instillations.2 In this trial, 345 patients from 51 sites were randomized to the standard BCG schedule (6 weeks of induction followed by 3 weeks of maintenance at 3, 6, and 12 months: 15 instillations) versus the reduced frequency BCG schedule (induction at weeks 1, 2, and 6 followed by 2 weeks (week 1 and 3) of maintenance at 3, 6, and 12 months: 9 instillations). After 12 months of median follow-up, the intention-to-treat analysis showed a safety-relevant difference in recurrences between treatment arms: 46 of 170 in the reduced frequency arm versus 21 of 175 patients in the standard arm. As follows is the Kaplan-Meier curve depicting time to recurrence in the intention to treat analysis (hazard ratio [HR] 0.40, 95% confidence interval [CI] 0.24-0.68):

This trial clearly showed that a reduced frequency schedule was inferior to the standard schedule regarding the time to first recurrence and that standard dosing and timing should not be altered.
Nadofaragene firadenovec is a non-replicating recombinant type-5 adenovirus vector-based gene therapy that delivers a copy of the human IFNα2b gene. The Phase III trial of nadofaragene firadenovec for BCG unresponsive NMIBC was a multi-center study to investigate the safety and efficacy of intravesical nadofaragene firadenovec 75 mL once every 3 months in 157 patients with high-grade, BCG-unresponsive NMIBC.3 Cytology and cystoscopy (with biopsy if clinically indicated) were performed at 3, 6, and 9 months to evaluate for recurrence of high-grade disease. At 12 months, all patients underwent urine cytology, cystoscopy, and mandatory biopsy. Patients free from high-grade recurrence were eligible for retreatment at 3-month intervals while they remained high-grade recurrence free. The study met its primary endpoint with 53.4% of patients with carcinoma in situ (CIS) ±Ta/T1 achieving a complete response, all by 3 months, including 43.6% of these patients remaining free of high-grade recurrence at 15 months. As follows is the Kaplan-Meier curve for high-grade recurrence-free survival in the CIS +/- Ta/T1 arm: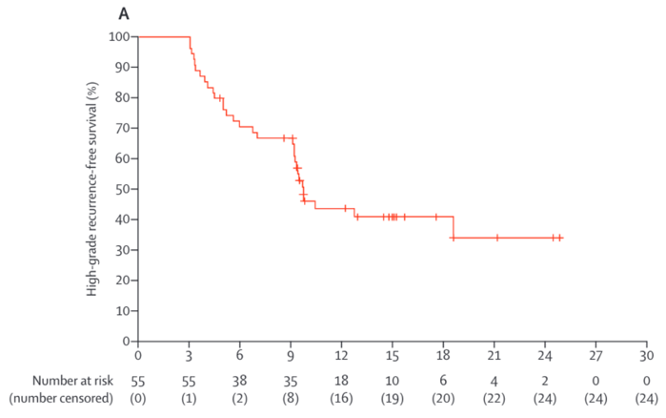
Moving to muscle-invasive bladder cancer, Dr. Seisen discussed the systematic review and individual patient data meta-analysis for patients undergoing radical cystectomy and enrolling in an ERAS® recovery pathway published in European Urology.4 This study included 22 articles reporting on 4,048 patients; individual patient data was obtained for 2,077 patients in 11 studies. ERAS® use was associated with reduced morbidity, quicker bowel recovery, and shorter length of stay, without affecting mortality. In multivariable models, length of stay was associated with ERAS® use (regression coefficient: -4.54, 95% CI -5.79 to -3.28 days with ERAS® p < 0.001) and Charlson Comorbidity Index (regression coefficient: +1.64, 95% CI 1.38-1.90 days for each point increase, p < 0.001), and varied between hospitals (from regression coefficient: -1.59, 95% CI -3.03 to -0.14 to regression coefficient: +4.55, 95% CI 1.89-7.21 days, p < 0.03). As such, this study showed that enhanced recovery in patients undergoing radical cystectomy is associated with fewer surgical complications and a shorter hospital stay and should be utilized post-operatively whenever feasible.
Arguably the most important and practice-changing study in 2020 in the metastatic urothelial carcinoma disease space was data reported from the Javelin Bladder 100 trial.5 In this Phase III trial, the trialists randomly assigned 700 patients with unresectable locally advanced or metastatic urothelial cancer who did not have disease progression with first-line chemotherapy (four to six cycles of gemcitabine plus cisplatin or carboplatin) to receive the best supportive care with or without maintenance avelumab. Javelin Bladder 100 found that overall survival at 1 year was 71.3% in the avelumab group and 58.4% in the control group (median overall survival, 21.4 months versus 14.3 months; HR for death 0.69, 95% CI 0.56 to 0.86; p = 0.001):
Avelumab also significantly prolonged overall survival in the PD-L1-positive population: overall survival at 1 year was 79.1% in the avelumab group and 60.4% in the control group (HR 0.56, 95% CI 0.40 to 0.79; p<0.001). This immediately became the standard of care in those patients with an appropriate response to front-line chemotherapy.
Switching to upper tract urothelial carcinoma, Dr. Seisen discussed the Phase III OLYMPUS study.6 This was an open-label, single arm trial designed to assess the efficacy, safety, and tolerability of UGN-101 in patients with low grade, noninvasive upper tract urothelial cancer. Patients were accrued at 24 academic sites in the United States and Israel. Eligible patients had either primary or recurrent biopsy-proven low-grade upper tract urothelial carcinoma of the renal pelvis or calyces, diagnosed in the two months prior to trial screening. Enrolled patients received six once-weekly instillations of UGN-101 as an induction course. Four to six weeks following initial treatment, patients received their primary disease evaluation including ureteroscopy, selective upper tract cytology, and for-cause biopsy where indicated. Complete response was defined as a negative endoscopic evaluation and the absence of histologic or cytologic evidence of disease. Among 110 patients screening, 74 were enrolled and 71 patients received treatment. Additionally, among the 71 patients who received at least one dose, 42 patients (59%, 95% CI 47-71%) had a complete response at the time of primary disease evaluation. Of the remainder, 8 (11%) had a partial response, and 12 (17%) had no response. Twelve-month durability could be assessed in 20 patients, of which 14 (70%) showed ongoing durability of their complete response.
Finally, Dr. Seisen discussed the practice-changing POUT trial in upper tract urothelial carcinoma.7 This was a Phase III, parallel-group, open-label, randomized controlled trial done at 71 National Health Service (NHS) hospitals in the United Kingdom. Eligible patients who had received a radical nephroureterectomy for upper tract urothelial carcinoma were postoperatively staged with either muscle-invasive (pT2–pT4, pNany) or lymph node-positive (pTany, pN1–3) M0 disease, and were fit to receive adjuvant chemotherapy within 90 days of surgery. The primary endpoint of this trial was disease-free survival (DFS) defined as time from randomization to either first recurrence in the tumor bed, first metastasis, or death from any cause. There were 261 patients included in POUT, including 129 patients randomized to surveillance and 132 to chemotherapy. There were 60 (47%) DFS events in the surveillance cohort and 35 (27%) in the chemotherapy cohort, corresponding to a unadjusted HR of 0.45 (95% CI 0.30-0.68) in favor of chemotherapy (log-rank p = 0.0001):
Additionally, the three-year DFS rate was 46% for surveillance (95% CI 36-56) and 71% for chemotherapy (95% CI 61-78). Metastasis free survival (MFS) also favored chemotherapy, with a HR of 0.48 (95% CI 0.31-0.74, log-rank p = 0.0007), and the three-year event-free rates were 53% (95% CI 42-63) for those on surveillance and 71% (95% CI 60-79) for those receiving chemotherapy:
Following this trial, adjuvant chemotherapy was considered the standard of care after nephroureterectomy for this high-risk patient population.
Presented by: Thomas Seisen, MD, MSc, Assistant Professor of Urology, Pitié Salpêtrière Hospital, Sorbonne University, Paris, France
Written by: Zachary Klaassen, MD, MSc, Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, Georgia, Twitter: @zklaassen_md during the 18th Meeting of the EAU Section of Oncological Urology (ESOU21), January 29-31, 2021
References:
1. Lindgren, Maria S., Peter Bue, Nessn Azawi, Linea Blichert-Refsgaard, Maria O. Sundelin, Lars Dyrskjøt, and Jørgen B. Jensen. "The DaBlaCa-13 Study: Short-term, Intensive Chemoresection Versus Standard Adjuvant Intravesical Instillations in Non–muscle-invasive Bladder Cancer—A Randomised Controlled Trial." European Urology 78, no. 6 (2020): 856-862.
2. Grimm, M. O., van der Heijden, A. G., Colombel, M., Muilwijk, T., Martínez-Piñeiro, L., Babjuk, M. M., ... & Beardo, P. (2020). Treatment of High-grade Non–muscle-invasive Bladder Carcinoma by Standard Number and Dose of BCG Instillations Versus Reduced Number and Standard Dose of BCG Instillations: Results of the European Association of Urology Research Foundation Randomised Phase III Clinical Trial “NIMBUS”. European Urology, 78(5), 690-698.
3. Boorjian, Stephen A., Mehrdad Alemozaffar, Badrinath R. Konety, Neal D. Shore, Leonard G. Gomella, Ashish M. Kamat, Trinity J. Bivalacqua et al. "Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial." The Lancet Oncology (2020).
4. Williams, Stephen B., Marcus GK Cumberbatch, Ashish M. Kamat, Ibrahim Jubber, Preston S. Kerr, John S. McGrath, Hooman Djaladat et al. "Reporting radical cystectomy outcomes following implementation of enhanced recovery after surgery protocols: a systematic review and individual patient data meta-analysis." European urology (2020).
5. Powles, Thomas, Se Hoon Park, Eric Voog, Claudia Caserta, Begoña P. Valderrama, Howard Gurney, Haralabos Kalofonos et al. "Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma." New England Journal of Medicine 383, no. 13 (2020): 1218-1230.
6. Kleinmann, Nir, Surena F. Matin, Phillip M. Pierorazio, John L. Gore, Ahmad Shabsigh, Brian Hu, Karim Chamie et al. "Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial." The lancet oncology 21, no. 6 (2020): 776-785.
7. Birtle, Alison, Mark Johnson, John Chester, Robert Jones, David Dolling, Richard T. Bryan, Christopher Harris et al. "Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial." The Lancet 395, no. 10232 (2020): 1268-1277.


