(UroToday.com) The must read, best of 2020 papers in testis and penile cancer lecture was provided by Dr. Pia Paffenholz from Cologne, Germany at the European Association of Urology (EAU) Section of Oncological Urology (ESOU) 2021 virtual meeting Dr. Paffenholz presented two papers relating to testis cancer and one relating to penile cancer.
miR371 is a biomarker for viable germ cell tumor, however, it has been shown to be undetectable in teratoma-only patients and thus there is a “teratoma gap”. miR375 may act as a potential predictive biomarker for teratoma. The first paper presented by Dr. Paffenholz was by Nappi et al. entitled “Integrated Expression of Circulating miR375 and miR371 to Identify Teratoma and Active Germ Cell Malignancy Components in Malignant Germ Cell Tumors” published in European Urology.1 The objective of this study was to define miR375 alone or integrated with miR371 as a predictive biomarker of teratoma and vital carcinoma. This was a multi-center study including 100 patients with testicular germ cell tumor divided into a discovery cohort (62 patients – teratoma on post-chemotherapy RPLND, CSI on surveillance, or metastatic seminoma prior to chemotherapy) and a validation cohort (38 post-chemotherapy patients – teratoma/vital carcinoma in post-chemotherapy RPLND, complete response). The primary endpoint was AUC in detecting teratoma for miR375 and miR375/miR371. The area under the receiver operating characteristic curve values for miR375, miR371, and miR371-miR375 were, respectively, 0.93 (95% CI 0.87-0.99), 0.59 (95% CI 0.44-0.73), and 0.95 (95% CI 0.90-0.99) in the discovery cohort and 0.55 (95% CI 0.36-0.74), 0.74 (95% CI 0.58-0.91), and 0.77 (95% CI 0.62-0.93) in the validation cohort:
Dr. Paffenholz notes that the presence of miR371 indicates active germ cell tumor, while the absence of miR371 and the overexpression of miR375 suggest the presence of teratoma in absence of active germ cell tumor components. Thus, the combination of plasma miR375-miR371 shows promise in detecting teratoma, but larger studies are warranted.
The second testicular cancer paper discussed by Dr. Paffenholz also related to miRNA by Lobo et al. entitled “Utility of Serum miR-371a-3p in Predicting Relapse on Surveillance in Patients with Clinical Stage I Testicular Germ Cell Cancer” from the Princess Margaret Cancer Centre and published in European Urology Oncology.2 The objective of this study was to explore the association between serum miR-371a-3p and CSI surveillance relapse. This study included banked serum from 151 CSI patients (101 seminoma, 50 NSGCT), of which 34 (23%) of patients suffered disease relapse. There was no association between post-orchiectomy miR-371a-3p (2.43 vs 2.74, p = 0.31) or percent decline from before to after orchiectomy (95.8% vs 93.1%, p = 0.14) and relapse. However, post-orchiectomy miR-371a-3p levels rose as the date of miRNA assessment approached relapse, and at relapse serum markers alpha-fetoprotein and human chorionic gonadotropin were normal in 62% of patients but miR-371a-3p was elevated in 94.1%. As follows is the relationship between the proximity of post-orchiectomy miR-371a-3p assessment and eventual relapse:

Dr. Paffenholz summarized this study noting that serum post-orchiectomy miR371 levels were not associated with relapse in CSI patients and that classical histological markers (tumor size, LVI, EC%) were more reliable. Given that miR371 levels were consistently elevated at relapse and correlated with burden of relapse, it is possible that miR371 is a potential early non-invasive relapse marker. Universal guidelines are needed to standardize the method of isolation, detection, quantification, and reporting of miR371 levels (plasma versus serum, normalization, and cutoff values).
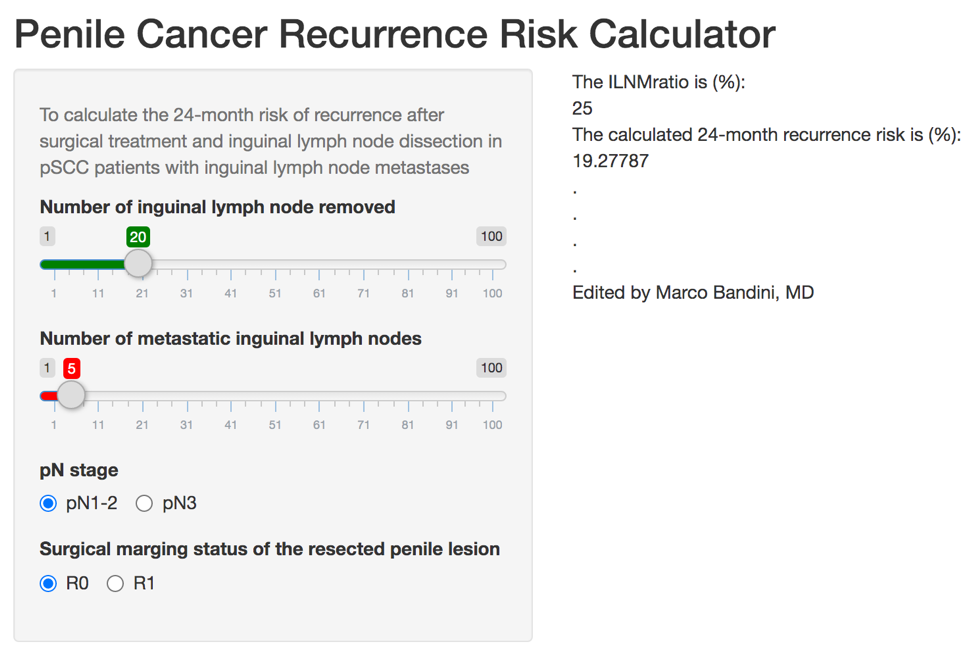
Patients with penile squamous cell carcinoma and inguinal lymph node metastases are lymphadenectomy have a dismal prognosis. As such, the EAU guidelines offer a strong rating for offering patients with pN2-N3 tumors adjuvant chemotherapy after radical lymphadenectomy (ie. 3-4 cycles of cisplatin, a taxane plus 5-fluorouracil or ifosfamide). However, the evidence supporting adjuvant chemotherapy in pN1 or adjuvant radiotherapy is weak. The final study presented by Dr. Paffenholz was by Bandini and colleagues entitled “Optimizing the selection of candidates for neoadjuvant chemotherapy amongst patients with node-positive penile squamous cell carcinoma” and published in BJU International3. The objective of this study was to identify predictors of poor overall survival amongst patients with penile squamous cell carcinoma with clinical inguinal lymphadenopathy, in order to define the best candidates for neoadjuvant chemotherapy. This study utilized an international, multicenter database of 924 patients with penile squamous cell carcinoma, we identified 334 men who harbored clinical inguinal lymphadenopathy. There were 120 (35.9%) cN1 patients, 152 (45.5%) cN2 patients, and 62 (18.6%) with cN3 disease. The median overall survival was 107 months, with a 24-month overall survival rate of 66%. Using regression-tree analysis (area under the curve = 70%), patients with cN3 and cN2 with PET/CT-detected inguinal and pelvic nodal activity had a higher risk of 24-month mortality (>50%), and neoadjuvant chemotherapy was associated with improved 24-month overall survival rates (54% vs 33%) only in this subgroup of patients (p = 0.002). As follows are the Kaplan-Meier curves for overall survival according to use of neoadjuvant chemotherapy for eligible (left) and ineligible (right) patients:

Based on this paper, there is a now a risk score for prediction of recurrence in patients with penile squamous cell carcinoma with inguinal lymph node metastases: https://marco-bandini-md-sanraffaele.shinyapps.io/pcrrc
Presented by: Pia Paffenholz, MD, Urologist, University Hospital Cologne, Cologne, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md during the 18th Meeting of the EAU Section of Oncological Urology (ESOU21), January 29-31, 2021
References:
1. Nappi L, Thi M, Adra N, et al. Integrated Expression of Circulating miR375 and miR371 to Identify Teratoma and Active Germ Cell Malignancy Components in Malignant Germ Cell Tumors. Eur Urol. 2021 Jan;79(1):16-19.
2. Lobo J, Leao R, Gillis AJM, et al. Utility of Serum miR-371a-3p in Predicting Relapse on Surveillance in Patients with Clinical Stage I Testicular Germ Cell Cancer. Eur Urol Oncol2020 Dec 4 [Epub ahead of print].
3. Bandini M, Albersen M, Chipollini J, et al. Optimizing the selection of candidates for neoadjuvant chemotherapy amongst patients with node-positive penile squamous cell carcinoma. BJU Int2020 Jun;125(6):867-875.


