(UroToday.com) The 2022 EAU Section of Oncological Urology (ESOU) Annual Meeting included a session on locally advanced and metastatic renal cancer and a presentation by Dr. Laurence Albiges discussing future directions of systemic treatment for metastatic renal cell carcinoma (RCC), specifically what to expect over the next 5 years.
Dr. Albiges notes that understanding tumor biology defines our novel therapeutic options. HIF2alpha inhibition (MK6482, belzutifan) was initially tested in a phase I/II trial (n=55), with patients receiving a median of 3 lines of prior therapy (67% VEGF and PD1 inhibitors) and with a median follow-up of 27.7 months. This trial had an objective response rate of 25%, disease control rate of 80%, and median progression free survival of 14.5 months. Of note, the safety profile of belzutifan was distinct from VEGF/VEGFR inhibitors, including anemia (76% any grade, 27% grade 3), and hypoxia (31% any grade, 16% grade 3).
The inhibition of HIF-2alpha trial assessing Belzutifan in patients with VHL was recently published in the New England Journal of Medicine.1 This phase 2 open-label trial investigated the efficacy and safety of the belzutifan administered orally at a dose of 120 mg daily, in patients with renal cell carcinoma associated with VHL disease. The primary endpoint was objective response (complete or partial response) and other assessments included responses to belzutifan in patients with non–renal cell carcinoma neoplasms and the safety of belzutifan. Over a median follow-up of 21.8 months (range, 20.2 to 30.1 months), the percentage of patients with RCC who had an objective response was 49% (95% CI, 36 to 62). There were no complete responses, but 49% of patients had partial response, 49% had stable disease, and only 3% of patients had progressive disease. Remarkably, a reduction in the sum of all target lesion diameters was observed in 56 patients (92%):
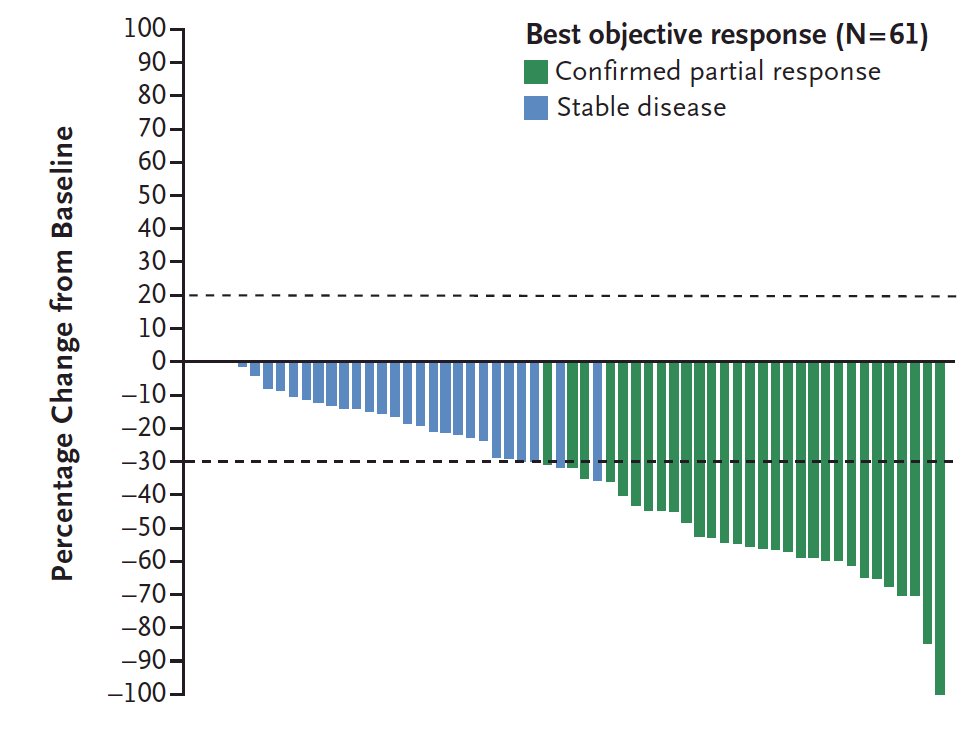
Most patients had growing tumors before treatment, followed by an observed reduction in the sum of the largest tumor diameters after treatment began:
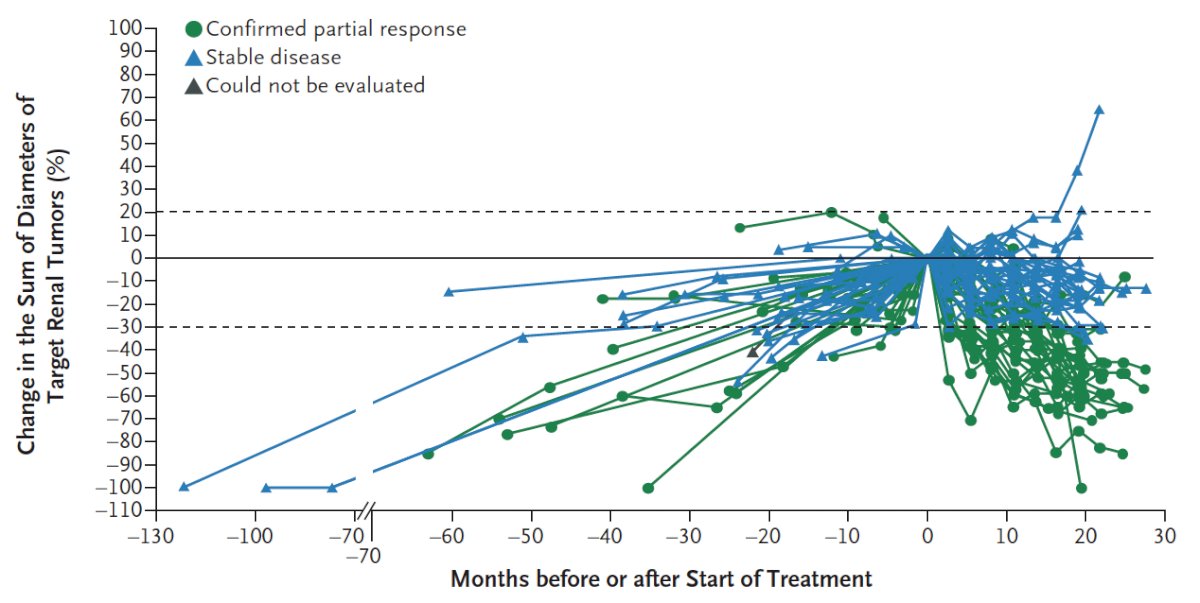
The median time to response was 8.3 months, PFS at 24 months was 96%, 89% of patients were still on therapy at the time of data analysis, and median duration was not reached.
Currently, there is a phase 3 trial of single agent MK-6482 versus everolimus (NCT04195750) ongoing among patients receiving <=3 systemic regimens, with the following trial schema:

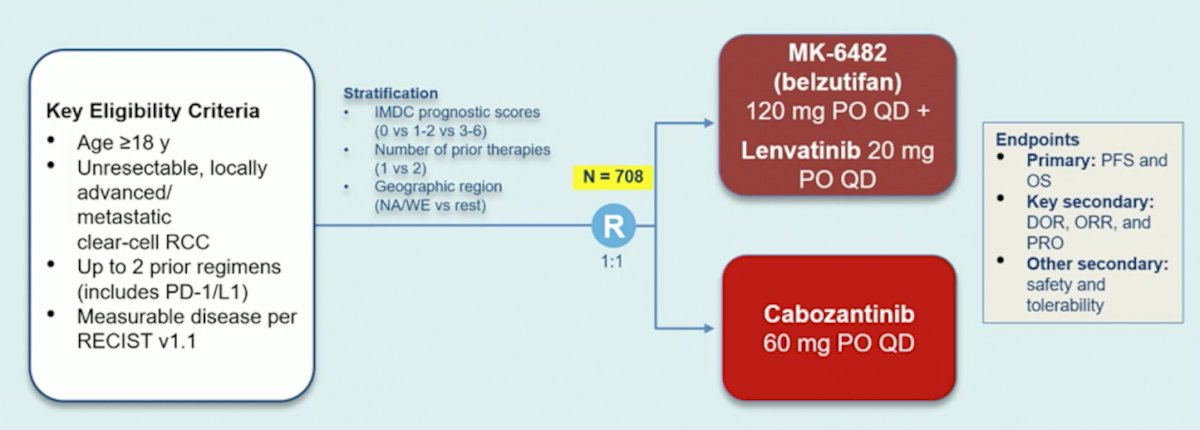
HIF2alpha inhibition has also been tested in combination studies, including PT2385 + nivolumab in 50 patients, reporting an objective response rate of 22%, a disease control rate of 64%, and a median progression free survival of 7.3 months. Among 52 patients over a median follow-up of 11.3 months, belzutifan + cabozantinib showed an objective response rate of 22% and disease control rate of 90%. Among patients that have had up to 2 prior regimens (including PD-1/L1), a phase 3 trial is ongoing testing belzutifan + lenvatinib versus cabozantinib, with the trial schema as follows:
Dr. Albiges notes that there are clinical implications for enhancing the immune response in understanding the biology of clear cell RCC. This may open up a new area of therapeutics including immune checkpoint blockade, cell therapy, and personalized vaccine use. There are many potential candidates for modulating the immune response, as highlighted in the following figure:
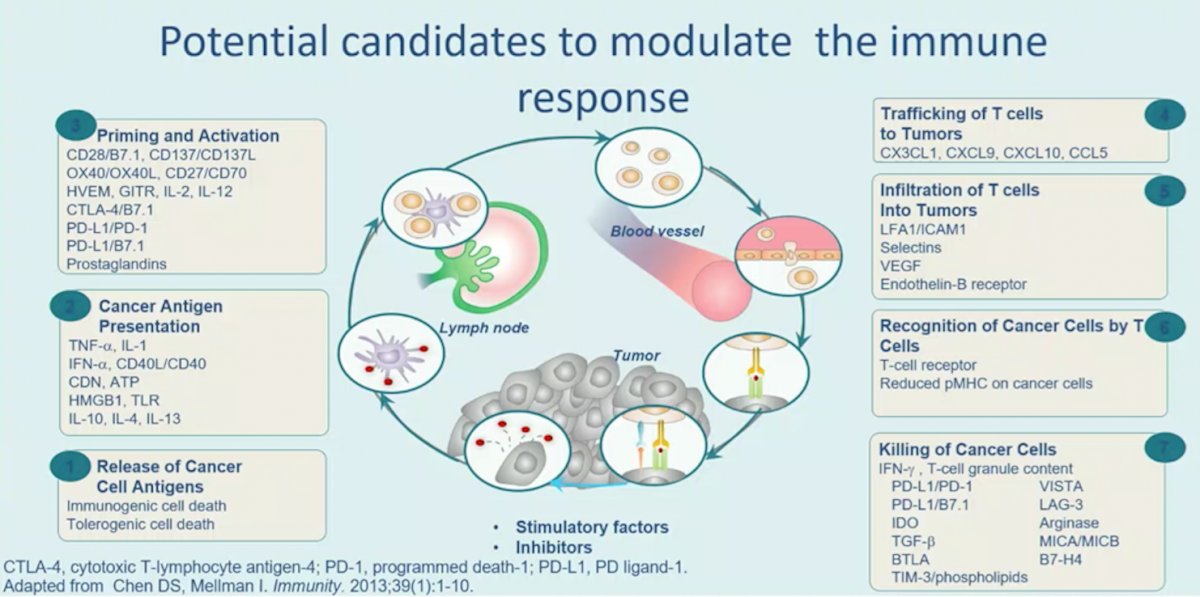
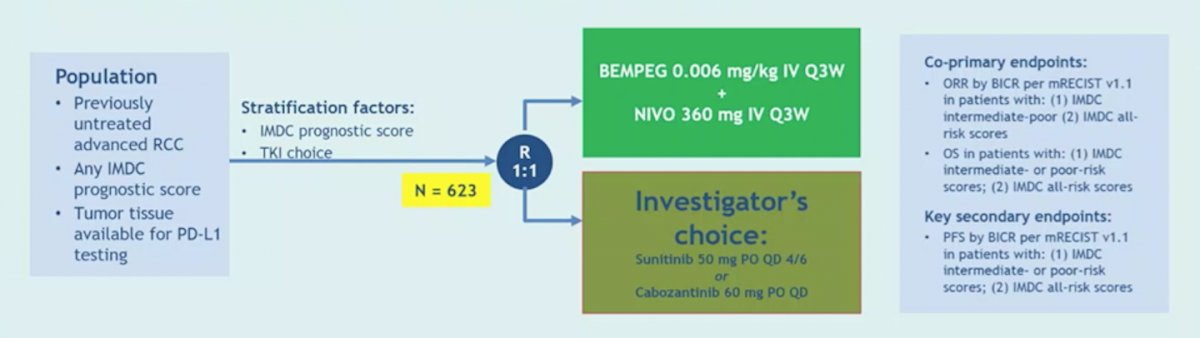
Potential candidates for modulating the immune response include NKTR-214 (bempegaldesleukin), a PEGylated interleukin-2 (IL-2) acting as a CD122-preferential IL-2 pathway agonist. In the PIVOT-09 (NCT03729245) trial, bempegaldesleukin + nivolumab will be tested against sunitinib or cabozantinib in the first-line setting, with the trial schema as follows:
Dr. Albiges notes that a potentially new immune checkpoint blockade target for metastatic RCC is tiragolumab, which has been granted breakthrough designation by the US Food and Drug Administration, in combination with atezolizumab for the first-line treatment of patients with metastatic NSCLC whose tumors have high PD-L1 expression based on the phase II CITYSCAPE trial showing prolonged PFS. Additionally, although early, chimeric antigen receptor T cell therapy (autologous CAR-T) has emerged in metastatic RCC, to date study sample sizes remain small (phase I trials, n = ~5).
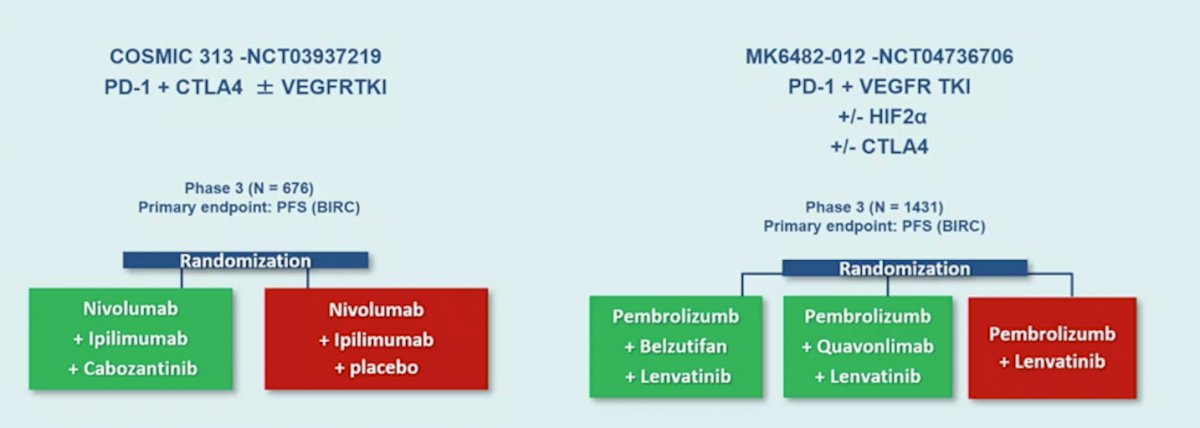
To conclude, Dr. Albiges also highlighted novel therapeutic approaches beyond drugs. First, this includes treatment intensification in the first line by way of triplet therapy, focusing on two trials: COSMIC 313 (PD-1 + CTLA4 +/- VEGFR-TKI) and MK6482 (PD-1 + VEGFR-TKI, +/- HIF2alpha +/- CTLA4):
Second, is a treatment-tailed approach, specifically aiming at de-escalation. The multicenter European TITAN-RCC study enrolled 258 first-line and second-line (after TKI) patients with IMDC intermediate and poor-risk, advanced clear cell RCC between October 2016 and December 2018. Patients started with nivolumab 240 mg Q2W induction. Patients with early significant progressive disease at week 8 or either stable disease or progressive disease at week 16 received 2-4 nivolumab + ipilimumab boost cycles. Responders (defined as either partial response or complete response) to nivolumab monotherapy continued with maintenance with nivolumab + ipilimumab boosts only for progression.
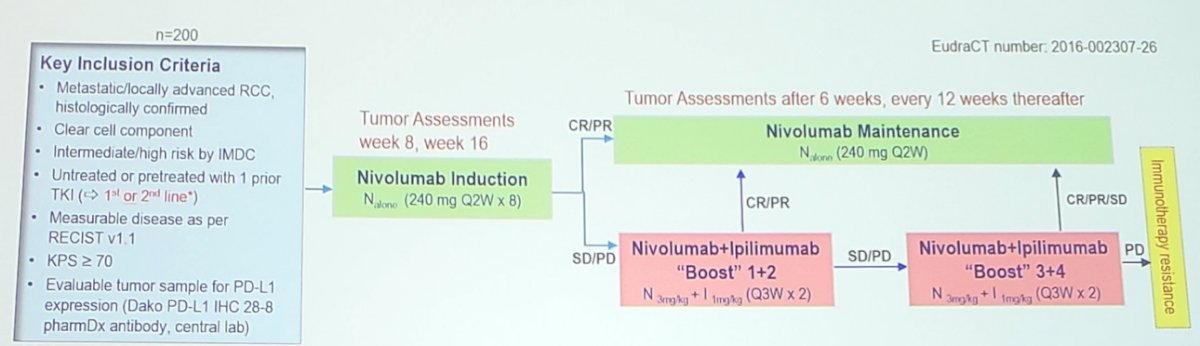
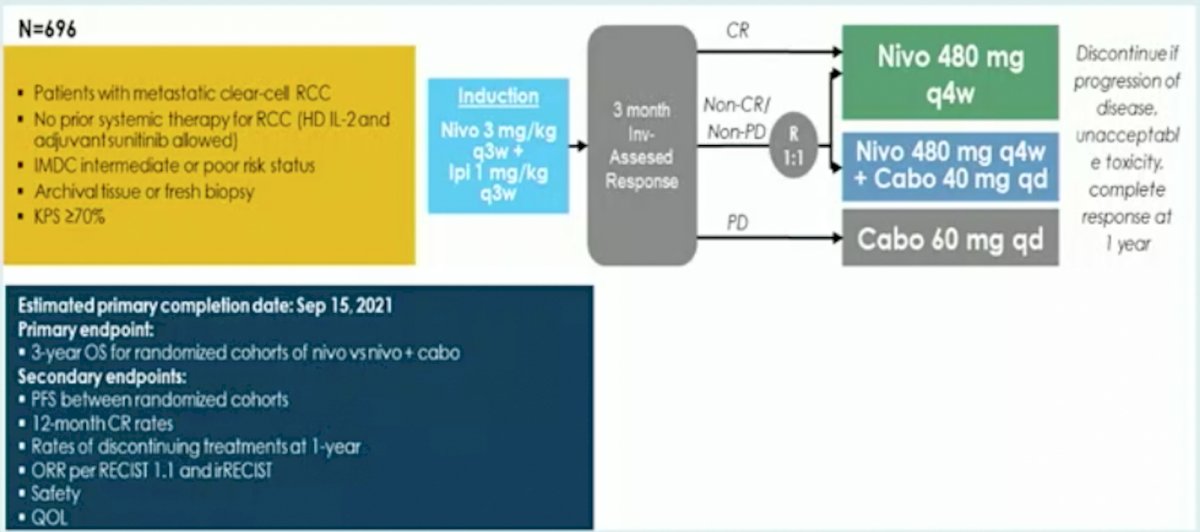
The primary endpoint was confirmed investigator assessed objective response rate (ORR) per RECIST independent in first and second line. Secondary endpoints include activity of nivolumab monotherapy, remission rate with nivolumab + ipilimumab boosts, safety, overall survival and quality of life. As has been presented at previous meetings, unfortunately, there is limited activity of an ipilimumab rescue strategy in these patients (10%). Dr. Albiges notes that we await the results of the pending PDIGREE trial, whereby patients will be induced with nivolumab + ipilimumab followed by tailoring of their treatment based on response:
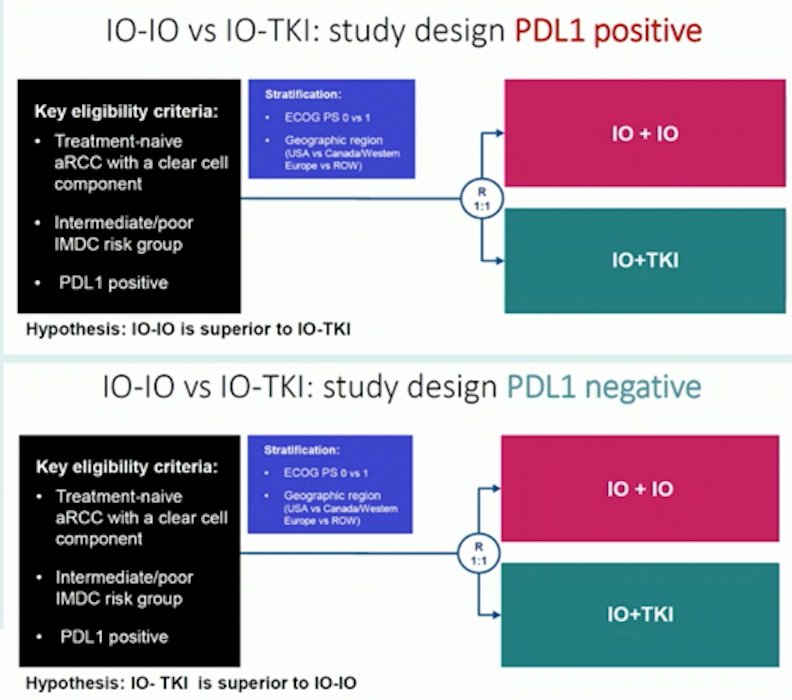
Third, is the holy grail of biomarker selection for upfront combination therapy. The BIONIKK trial showed that biomarker trials are feasible, however, we still need to define selection at the patient level in order to assign the best combination on a routine basis. Based on PD-L1 status, several hypotheses driven trials are being considered, as highlighted by Dr. Albiges:
Finally, with regards to novel therapeutic approaches beyond drugs, is the host, specifically gut microbiota as a therapeutic approach. Gut bacteria composition drives primary resistance to cancer immunotherapy in RCC. What has been previously shown is that fecal microbiota transplantation of stool samples from RCC patients into mice dictates outcomes after PD-1 blockade. Dr. Albiges notes that interventional studies are ongoing to modulate the gut microbiome in patients with metastatic RCC receiving checkpoint inhibition and to assess the impact of clinical outcomes.
Dr. Albiges concluded her presentation discussing future directions in treatment for metastatic RCC over the next 5 years with the following take-home messages:
- Adjuvant therapy integration into our clinical practice will be important
- New agents, including HIF 2alpha inhibition, are under evaluation
- There are going to be new combinations tested over the next several years, including a potential role for triplet therapy
- Currently, there are insufficient biomarker driven trials
- We are in need of new algorithms focusing on PD-1 resistance strategies
Presented by: Laurence Albiges, MD, PhD, Medical Oncology, Institut Gustave Roussy, Villejuif, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 EAU Section of Oncological Urology (ESOU) Hybrid Annual Meeting, Madrid, Spain, Fri, Jan 21 – Sun, Jan 23, 2022.
References


