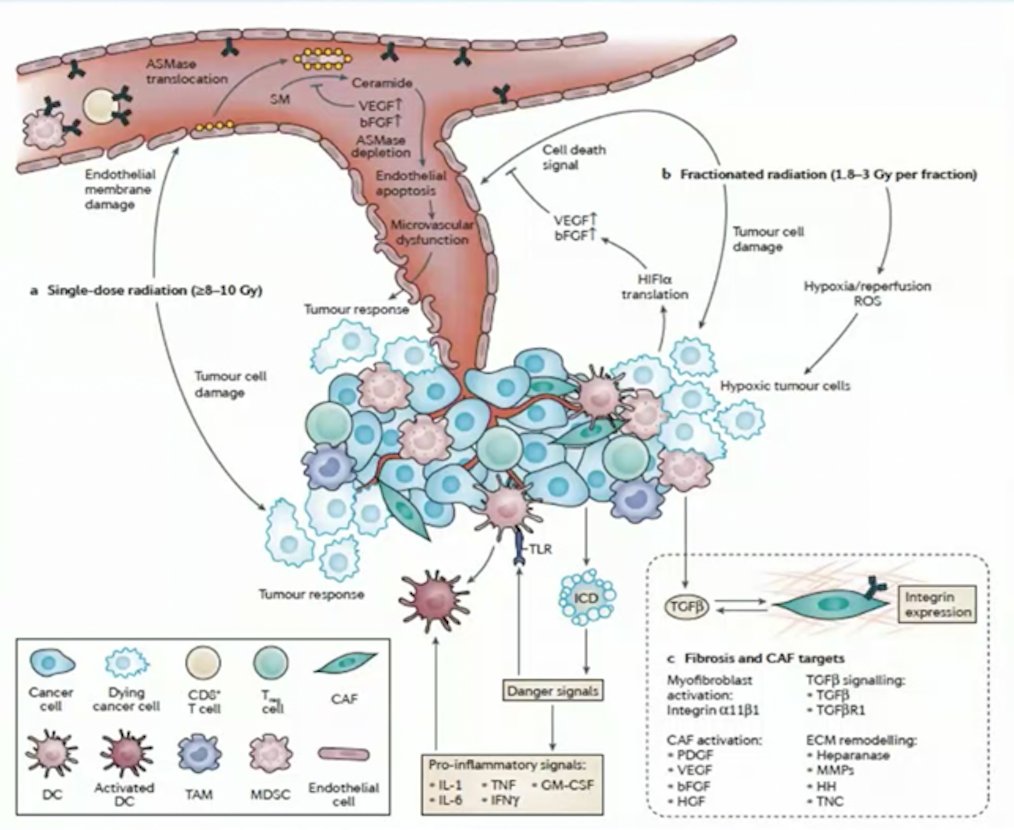
(UroToday.com) The 2022 EAU Section of Oncological Urology (ESOU) Annual Meeting included a session on locally advanced and metastatic renal cancer and a presentation by Dr. Shankar Siva discussing the role of stereotactic ablative body radiotherapy (SABR) for kidney cancer. Dr. Siva notes that renal cell carcinoma (RCC) has historically been considered a radioresistant disease. Looking at data from the mid-1980s, among 125 patients with metastatic RCC treated for palliation, doses ranging from 20 Gy – 60 Gy achieved a 65% response rate. However, over the last several years, the dogma of RCC being radioresistant has been challenged with improving technology and therapeutics. Preclinical studies from the mid-1990s suggested that a small fraction of cells are killed at doses of 2 Gy, whereas there is logarithmic cell death at doses of > 6 Gy. Dr. Siva notes that a different mechanism of cell kill is likely observed with high-dose radiation (ie. SABR), including endothelial apoptosis, leading to ceramide/sphingomyelinase induced cell death, and pro-inflammatory signaling for adaptive immunity:1
A meta-analysis of 28 studies published in 2019 showed that 1,602 mutually exclusive patients were treated with SABR for oligometastatic RCC, including 679 extracranial and 923 intracranial lesions.1 The median treatment volume was 59.7 cc for extracranial (IQR 31.1-71.4) and 2.3cc for intracranial (IQR 1.3-4.3) lesions. Using a random-effect model, the summary effect size for 1-year local control was 89.1% (95% CI 83.6-93.7%, I2=71%) and 90.1% (95% CI 83.5-95.3%, I2=74%) for extracranial and intracranial disease, respectively. The 1-year survival rates were 86.8% (95% CI 62-99.8%, I2=95%) and 49.7% (95% CI 41.1-58.3%, I2=74%) for extracranial and intracranial disease, respectively.
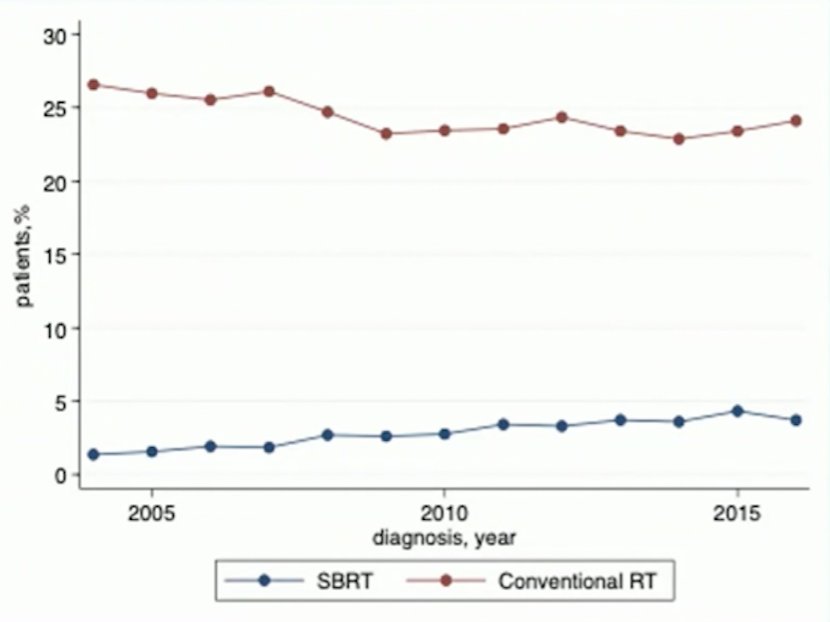
Work from Dr. Choueiri’s group looking at the National Cancer Database showed that among 65,345 patients diagnosed with metastatic RCC, 1,919 patients (2.9%) in the TKI era (2004-2016) had SBRT, compared to 15,871 (24.3%) receiving conventional radiotherapy, with a significant increase in the use of SBRT over time:
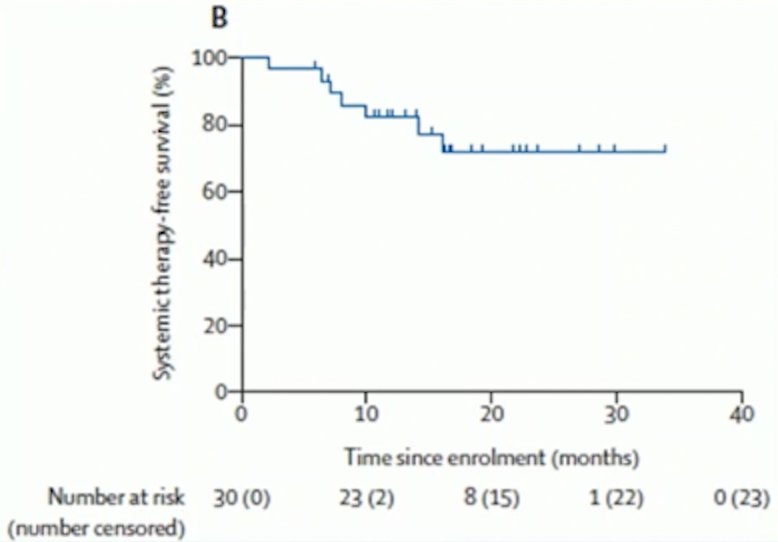
Importantly, Dr. Siva notes that SBRT is listed in the NCCN guidelines for oligometastatic RCC for both patients with clear cell and non-clear cell histology. Over the last couple of years, there have been three important trials in the treatment landscape of SABR for oligometastatic RCC, all of which were subsequently highlighted by Dr. Siva. First, Tang et al.4 assessed definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma in a single-arm, single-centre, feasibility, phase 2 trial from MD Anderson. Among 30 patients with a median number of metastasis of 1, patients were treated with SABR (defined as ≤5 fractions with ≥7 Gy per fraction) to all lesions and maintained off systemic therapy. When lesion location precluded safe SABR, patients were treated with hypofractionated intensity-modulated radiotherapy regimes consisting of 60–70 Gy in ten fractions or 52.5–67.5 Gy in 15 fractions. The co-primary endpoints were feasibility (defined as all planned radiotherapy completed with <7 days unplanned breaks) and progression-free survival. All patients completed at least one round of radiotherapy with less than 7 days of unplanned breaks. At a median follow-up of 17.5 months (IQR 13.2–24.6), median progression-free survival was 22.7 months (95% CI 10.4–not reached) and 1-year progression-free survival 64% [95% CI 48–85]). The 1-year systemic-therapy free survival was 82%:
The second trial was a Canadian phase 2 trial assessing SABR with TKIs among 37 patients with 1-3 sites of oligoprogression.5 The median duration of TKI therapy prior to study entry was 18.6 months and the 1-year local control rate of the irradiated tumors was 93% (95% CI 71-98%). The median progression-free survival after SABR was 9.3 months (95% CI 7.5-15.7 months), and the cumulative incidence of changing systemic therapy was 47% (95% CI 32-68%) at 1 year, with a median time to change in systemic therapy of 12.6 months (95% CI 9.6-17.4 months). Finally, the 1-year OS rate was 92% (95% CI 82-100%) and there were no grade 3-5 SABR-related toxicities.
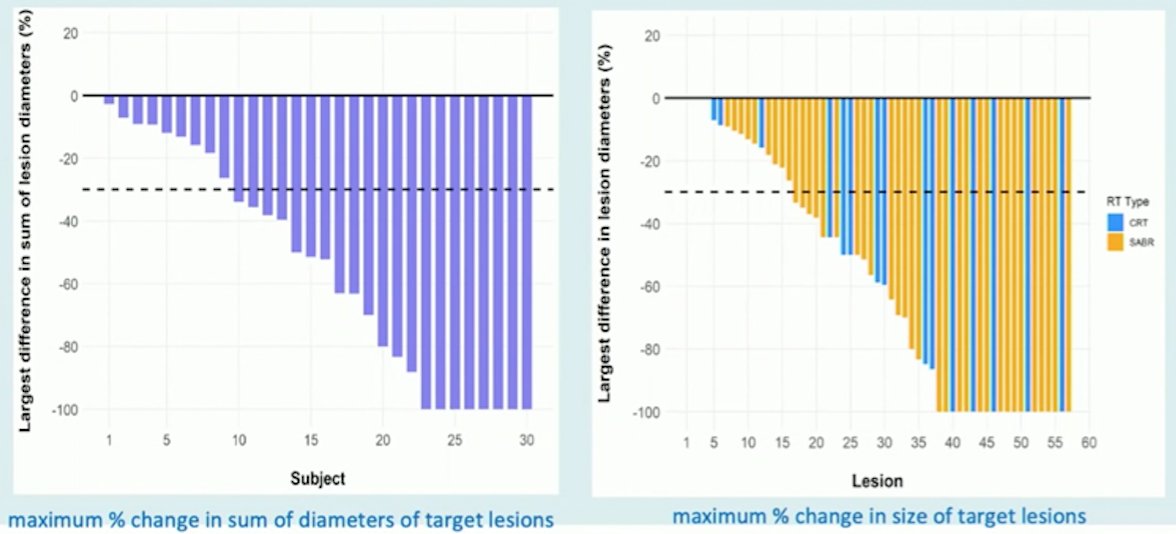
The final trial discussed by Dr. Siva was the recently published RAPPORT trial,6 a single-arm, multi-institutional phase I/II trial. The aim of this trial was to evaluate the safety and efficacy of total metastatic irradiation followed by short-course anti-programmed death receptor-1 immunotherapy in patients with oligometastatic clear cell RCC. Patients with two or fewer lines of prior systemic therapy and one to five oligometastases from clear cell RCC were eligible, and the intervention was a single fraction of 20 Gy SABR (or if not feasible, ten fractions of 3 Gy) given to all metastatic sites, followed by pembrolizumab 200 mg administered every 3 weeks for eight cycles. Among 30 patients, there were 44% of which had intermediate-risk and 56% with favorable-risk disease. Eighty-three oligometastases were irradiated (median three per patient), including eight adrenal, 11 bone, 43 lung, 12 lymph node, and nine soft tissue. Over a median follow-up of 2.3 years, four patients (13%) had grade 3 treatment-related adverse events: pneumonitis (n = 2), dyspnea (n = 1), and elevated alkaline phosphatase/alanine transaminase (n = 1), and there were no grade 4 or 5 adverse events. The freedom from local progression rate at 2 years was 92%, the objective response rate was 63%, and the disease control rate was 83%. The estimated 1- and 2-year OS rates were 90% and 74%, respectively, and for PFS was 60% and 45%, respectively. As follows is the maximum percent change in the sum of diameters of target lesions and the maximum percent change in the size of target lesions:
Dr. Siva’s take-away points from the RAPPORT trial included (i) the combination of SABR and short course of pembrolizumab in oligometastatic renal cell carcinoma is well tolerated with excellent control; (ii) the observed PFS of 15.6 months and objective response rate of 63% compares favorably to historic KEYNOTE-4277 pembrolizumab monotherapy (PFS of 7.1 months and objective response rate of 34%); (iii) durable responses and encouraging PFS were observed with this approach, which warrants further investigation.
Dr. Siva concluded his presentation of SABR for kidney cancer with the following take-home messages:
- Renal cell carcinoma is not radioresistant to ablative doses of radiation
- Prospective trials with SABR alone, oligoprogression, and a new frontier with combined SABR and immunotherapy in metastatic RCC is upon us
- SABR for oligometastatic RCC is guideline recommended and no longer experimental
Presented by: Shankar Siva, PhD, Peter MacCallum Cancer Center, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 EAU Section of Oncological Urology (ESOU) Hybrid Annual Meeting, Madrid, Spain, Fri, Jan 21 – Sun, Jan 23, 2022.
References:
- Siva S, Kothari G, Muacevic A, et al. Radiotherapy for renal cell carcinoma: renaissance of an overlooked approached. Nat Rev Urol. 2017;14(9):549-563.
- Zaorsky NG, Lehrer EJ, Kothari G, et al. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): A meta-analysis of 28 studies. Eur Urol Oncol 2019;2(5):515-523.
- Paciotti M, Schmidt AL, Ravi P, et al. Temporal trends and predictors in the use of Stereotactic Body Radiotherapy for treatment of metastatic renal cell carcinoma in the United States. The Oncologist. 2021 Mar 1 [Epub ahead of print].
- Tang C, Msaouel P, Hara K, et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: A single-arm, single-centre, feasibility, phase 2 trial. Lancet Oncol. 2021;22:1732-1739.
- Cheung P, Patel S, North SA, et al. Stereotactic Radiotherapy for Oligoprogression in Metastatic Renal Cell Cancer Patients Receiving Tyrosine Kinase Inhibitor Therapy: A Phase 2 Prospective Multicenter Study. Eur Urol. 2021 Dec;80(6):693-700.
- Siva S, Bressel M, Wood ST, et al. Stereotactic Radiotherapy and Short-course Pembrolizumab for Oligometastatic Renal Cell Carcinoma—The RAPPORT Trial. Eur Urol.
- McDermott DF, Lee JL, Ziobro M, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol. 2021 Mar 20;39(9):1029-1039.


