(UroToday.com) In a podium presentation in the Testis cancer Early detection and personalized management session at the 19th Meeting of the EAU Section of Oncological Urology, Dr. Fankhauser presented on the role of thromboprophylaxis in patients undergoing chemotherapy for metastatic germ cell tumors.
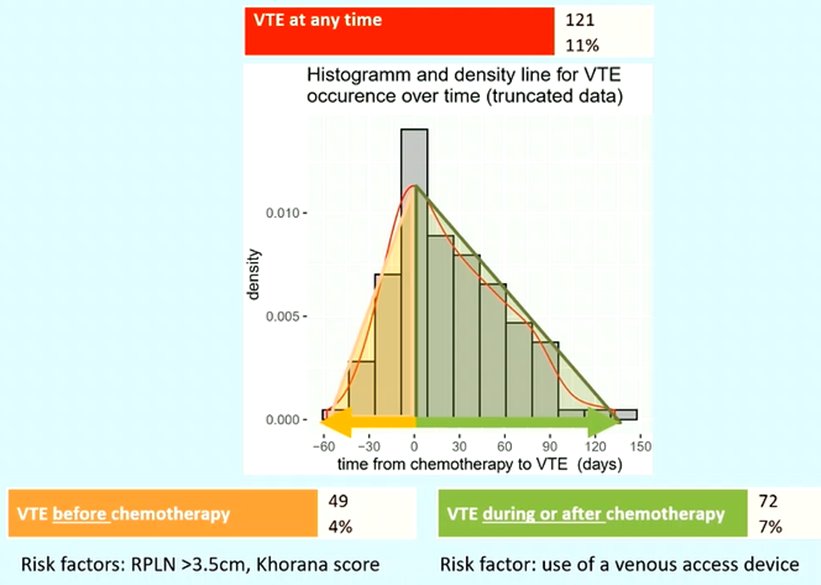
Dr. Fankhauser began by discussing the incidence of venous thromboembolism in patients undergoing first-line chemotherapy for metastatic germ cell tumors. Utilizing a cohort of 1120 men, they demonstrated that a total of 121 men (11%) had a venous thromboembolism event at any time. The greatest proportion of these events occurred around the time of chemotherapy initiation.
They further divided patients into those who had thromboembolic events prior to the start of chemotherapy and after the start of chemotherapy. Those that occur during or after chemotherapy likely reflect the effects of treatment and are potentially avoidable with the use of thromboprophylaxis while those that occur prior to the start of chemotherapy likely reflect the underlying biology.
During or after chemotherapy, 72 men (7%) experienced a thromboembolic event. The only identified risk factor for thromboembolism during this period was the presence of a central venous access device. In contrast, 49 patients (4%) had venous thromboembolism prior to the start of chemotherapy. A larger retroperitoneal mass (>3.5cm) and a higher Khorana score were associated with a higher likelihood of thromboembolism during this period.
Based on these data, Dr. Fankhauer concluded that there are relatively high rates of venous thromboembolism in patients before, during, and after chemotherapy for germ cell tumors. However, there are different risk factors during these time periods. He suggested that we should examine all staging CT scans for asymptomatic venous thromboembolism and consider a screening duplex ultrasound prior to initiating chemotherapy. Additionally, where deep venous thrombosis is identified, thromboprophylaxis should be identified early as most of these events occur around the time of chemotherapy initiation.
He further emphasized that a thromboembolic event may be life changed. Deep venous thrombosis can be associated with a post-thrombotic syndrome comprising pain and leg ulceration while pulmonary embolism may lead to death or chronic thromboembolic pulmonary hypertension. Further, patients that experience a thromboembolic event require long-term anticoagulation which may be life-long. This is associated with a non-trivial long-term risk of bleeding complications.
Thus, considering the role of thromboprophylaxis, Dr. Fankhauer then considered the available data. He highlighted two randomized controlled trials of oral anticoagulants in patients with cancer: the CASSINI trial and AVERT.

As a result of each of these studies demonstrated a significant decrease in rates of venous thromboembolism among patients receiving prophylaxis, ASCO recommends the use of thromboprophylaxis in patients who have an increased risk of venous thromboembolism and low bleed risk. However, very few patients with germ cell tumors were included in these trials. Thus, Dr. Fankhauer suggested that retrospective analyses may be helpful. However, given the need to account for a large number of relevant confounders, a large sample size would be required and a sufficient dataset is not available to provide confounding risk adjustment, not to mention the inability of these analyses to account for residual confounding.
Instead, he performed a simulation study, informed by the results of the AVERT study. Utilizing estimates of venous thromboembolism of 7% among all patients during or after chemotherapy, with increased rates among those with venous access devices (10%) and lower rates among though without such devices (5%), he then estimated the effect of thromboprophylaxis using the hazard ratio of 0.66 from the AVERT trial. Such extrapolation leads to estimates of an overall number needed to treat of 45 patients to avert one thromboembolic event, varying from 31 patients in those with venous access devices to 55 in those without. Notably, given the low baseline risk of bleeding events (1%), the number needed to treat to harm with respect to bleeding events is substantially higher (186).
These results informed recent EAU guidelines which suggest balancing individual patients' benefits and risks of thromboprophylaxis during first-line chemotherapy. Further, they recommend against the use of central venous access devices where feasible.
Presented by: Christian D. Fankhauser, MD MPH, PD Dr. med., Consultant Urologist, Lucerne, Switzerland

