The American Urologic Association guidelines state that clinicians must perform a bilateral PLND at time of surgery with curative intent. The minimum to be removed are the external, internal, and obturator LN (standard lymphadenectomy).
Disease specific survival is worsened when positive LN are present (figure 1). Mapping studies were performed demonstrating the incidence and location of positive LN (Figure 2), showing that almost all involved LN are included in the boundaries of the true pelvis. It is important to note the limitations of mapping studies, including the fact that the area to which a removed LN is assigned may vary from surgeon to surgeon. Additionally, it remains undetermined how many LNs are left behind and in which anatomic locations.
It has been shown that limited PLND removes only about 50% of all primary lymphatic landing sites. In order to remove 90%, PLND should be extended to include LNs lateral and medial to the internal iliac vessels, and the common iliac region up to the uretero-iliac crossing. In a meta-analysis of all studies comparing extended and standard PLND, overall odds ratio of 5-year recurrence-free survival rate was 1.63 (95% CI 1.28-2.07, p<0.001), suggesting a significant benefit for the extended PLND [1].
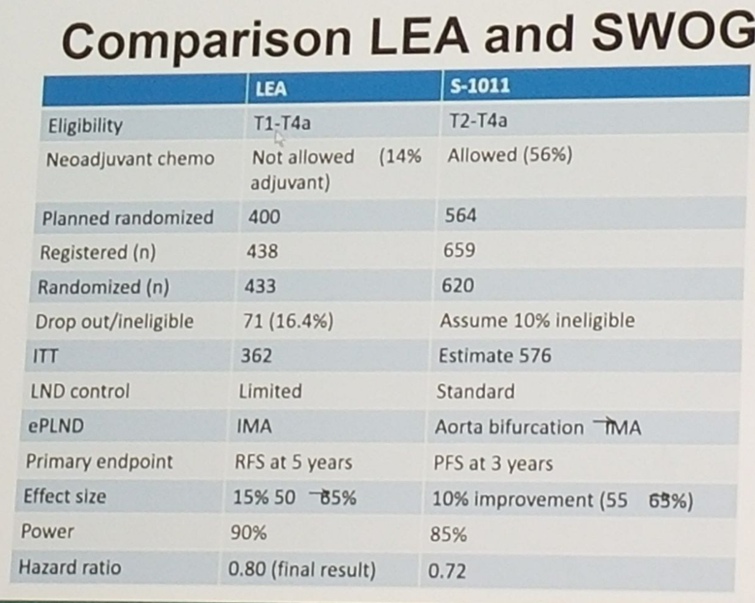
There are currently two phase 3 randomized trials trying to asses which PLND template is the most beneficial. These include the American SWOG 1011 (accrual complete, Pending) examining disease free survival of extended (including common iliac and presacral PLND) vs. standard PLND. This trial included all patients with predominant urothelial carcinoma of the bladder with T2-T4a disease and clinically node negative. The trial was open to a limited set of high volume and highly experienced surgeons and institutions in order to optimize accrual and evaluate the surgical quality monitoring plan. Surgeons included in the trial had to show a history of performing at least 50 cystectomies in the last 3 years and be working in a hospital that performs at least 30 cystectomies each year. Patients were randomized intra-operatively and intra-operative photos were required to assess dissection (figure 3).
The second phase 3 trial is the Association of Urogential Oncology and German Cancer Association (AUO AB25/02 [accrual complete, presented], the LEA trial. This trial examined progression free survival of extended PLND (up to the inferior mesenteric artery) vs. limited PLND. Figure 4 shows comparison of both trials.
Dr. Evans summarized his excellent talk by stating that LN metastasis occur in 25% of patients undergoing RC. Removal of the regional LNs at RC is important to optimize disease specific outcomes in BC patients. The extent of the PLND necessary remains controversial and evidence-based guidelines will require high level data from the prospective randomized trials.
Figure 1: Disease specific survival according to pathological N stage at Radical Cystectomy:
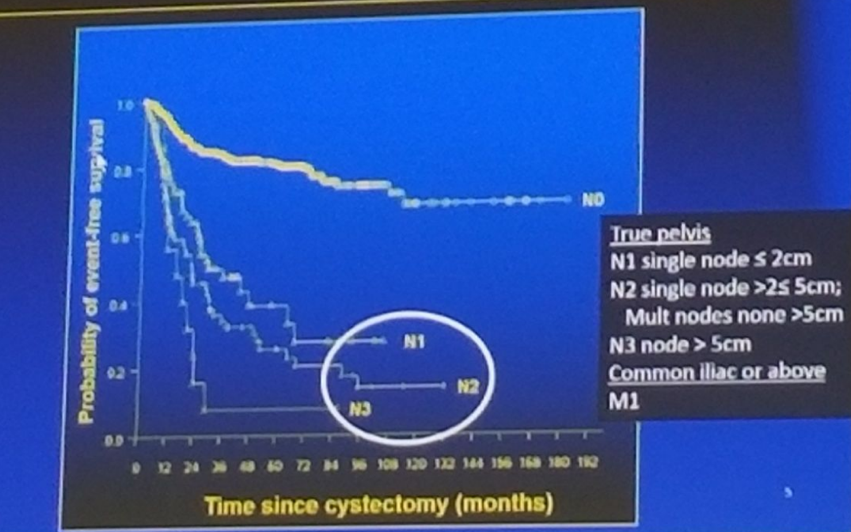
Figure 2: Mapping study of Pelvic Lymph nodes:
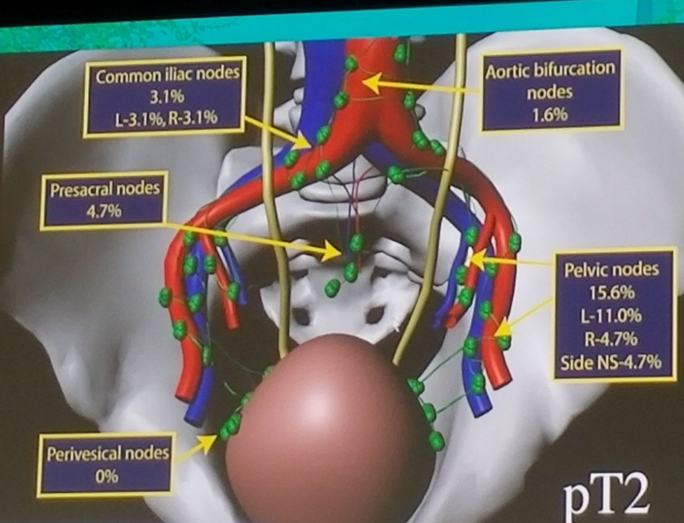
Figure 3: Intraoperative Photo of pelvic Lymph node dissection:

Figure 4:
Speaker: Christopher P. Evans, M.D., FACS, Chair, Department of Urology, Professor Urologic Oncology UC Davis Health, Sacramento California, USA
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at The 15th Meeting of the EAU Section of Oncological Urology ESOU18 - January 26-28, 2018 - Amsterdam, The Netherlands


