This work was presented at the European Association of Urology (EAU) meeting in Copenhagen in 2018. This was a Japanese prospective randomized controlled study, that randomized 250 patients with primary NMIBC to either TURBT and a single instillation of chemotherapy (Mitomycin), or to TURBT and continuous saline bladder instillation (2000 ml/hour for the 1st hour, 1000 ml/hour for 2 hours. And 500 ml/hour for fifteen hours). In all patients, no therapy was performed before any recurrence. The follow-up period included cystoscopy and cytology every 3-6 months, and the primary endpoint was recurrence-free survival (RFS). At a median follow-up of 45 months, no significant difference in progression-free survival and median time to 1st recurrence was noted between the two groups, as shown in Figure 1. Furthermore, there was a lower incidence of adverse events in the continuous saline bladder instillation group (8% vs. 35%, p<0.0001). The authors concluded that continuous saline instillation might be an alternative to single instillation of chemotherapy in NMIBC patients, regarding its prophylactic effect and safety.
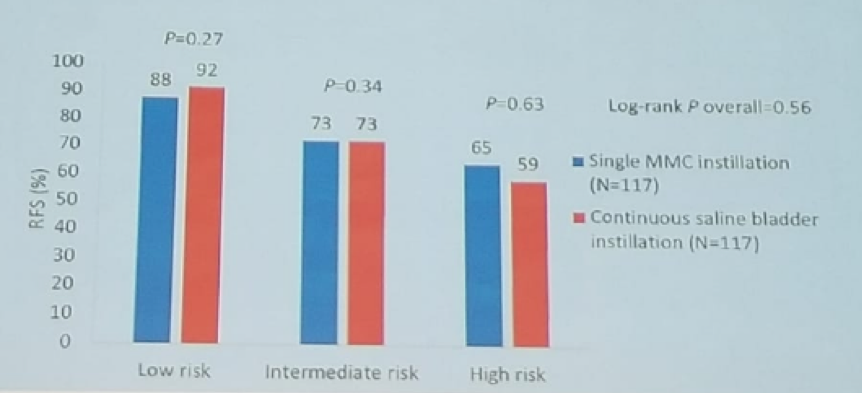
Figure 1- Study outcomes at a median follow-up of 45 months:
The next study discussed assessed which patients with NMIBC benefit from the immediate instillation of Mitomycin following TURBT. This Dutch prospective study was presented at the American Urologic Association (AUA) meeting in 2018 in San Francisco. 2 In this study, 1976 patients with intermediate or high-risk NMIBC were administered an immediate instillation following TURBT. Significant better recurrence-free survival rates were demonstrated in patients who underwent immediately vs. delayed instillation (hazard ratio 0.75, 95% C.I: 0.64-0.88, p<0.01). No difference was seen in treatment effect across patient subgroups. The authors concluded that an immediate instillation of Mitomycin seems to reduce the risk of recurrence in both intermediate and high-risk patients with NMIBC.
Next, Dr. Irani described a study assessing the role of chemoablation with Mitomycin in patients with recurrent low-risk NMIBC. This was the CALIBER study, which was a multi-center, phase 2, two-stage feasibility trial, presented at the EAU meeting. 3 This trial randomized patients with visually diagnosed recurrent low-risk NMIBC on flexible cystoscopy in the United Kingdom, into either surgery according to local practice, or chemoablation only without surgery, entailing four weekly outpatient intravesical instillation of 40 mg of Mitomycin. Three months following randomization, all patients had an outpatient cystoscopy and visual assessment of tumor response. If there was a complete visual response, a biopsy of the tumor bed was performed for confirmation. The primary endpoint was a complete response to chemoablation by visual assessment, and (if available) a biopsy at three months post-treatment. The feasibility of randomization demonstrated trial acceptance rates of 55% amongst eligible patients. The complete response rate (visual and histology if available) were 81% and 39% in the surgery and chemoablation arms, respectively. No grade 3-4 toxicities were witnessed in both arms. The authors, therefore, concluded, that although chemoablation seems feasible and safe, its low response rate does not warrant further investigation.
The next topic discussed was the incorporation of new agents in Bacillus Calmette–Guérin (BCG) unresponsive NMIBC patients. The assessment of Vicinium, an antibody fragment fused to a potent pseudomonas toxin was discussed in the VISTA trial. This was a single-arm, open label, multi-center phase 3 study presented at AUA in 20184. In this study BCG, unresponsive NMIBC patients were treated with intravesical instillation of 30 mg of Vicinium in 50 ml buffered saline held for 2 hours. The primary endpoint was complete response and duration of response. At three months follow-up the results showed a complete response in 39% of patients with CIS and papillary tumors that recurred within six months, and in 80% of patients with CIS and papillary tumors that recurred after six months but less than 11 months since receiving BCG. The safety profile was quite reassuring as well, with only four patients experiencing grade 3 or above complications. The authors concluded that Vicinium seems to be well tolerated and effective in patients with BCG unresponsive NMIBC at a follow-up of three months.
Next, Dr. Irani discussed a study assessing the role of radical cystectomy in elderly patients with muscle-invasive bladder cancer. This multi-center, retrospective database analysis was presented at the EAU in 2018 and included over 2000 patients who underwent radical cystectomy between 1990-2015. 5 Patients were stratified by age (75-79, 80-84, and >=85) and compared. At a median follow-up of 3.6 years, the death rate was 60%, 60%, and 70%, respectively, with cancer-specific death occurring in approximately half of the cases in all age groups. Therefore, the authors concluded that radical cystectomy remains a valid option in the elderly, even in patients older than 85.
The last study presented assessed whether long-acting anticholinergics are effective for nocturnal incontinence following radical cystectomy patients with orthotopic neobladders. This was an Egyptian prospective randomized placebo-controlled trial, that was presented at the EAU in 2018. 6 A total of 172 patients were included, randomized to either the long-acting tolterodine at a dosage of 4 mg at bedtime, or to a placebo group. After a 4-week period of treatment, and a two-week washout period, the treatment was switched among the groups, for a duration of another four weeks. Assessment of nocturnal enuresis was done at baseline, after four weeks, after washout, and at the end of the study. At a median follow-up of 79 months, the results demonstrated that 18-20% of patients became dry with tolterodine. The authors concluded the long-acting tolterodine seems effective for the treatment of nocturnal incontinence following radical cystectomy and orthotopic neobladder formation.
This was a most interesting discussion, providing bladder cancer highlights from the EAU and AUA in the last year, and Dr. Irani concluded his talk, looking forward to what will be presented in these meetings in 2019.
Presented by: Jacques Irani, MD, Hôpital Bicêtre, Hôpitaux Universitaires Paris-Sud
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the Global Conference on Bladder Cancer 2018 - September 20-21, 2018 Madrid, Spain
References:
1. Onishi T. et al. EAU 2018, abstract 735
2. Bosschieter J. AUA 2018, abstract PD66-07
3. Mostafid AH et al. EAU 2018, abstract 736
4. Dickstein R. et al. AUA 2018, abstract LBA-27
5. Pisano F et al. EAU 2018, abstract 709
6. Zahran M et al. EAU 2018, abstract 702


