The first briefly discussed topic was the use of enhanced cystoscopy – narrow band imaging vs. blue light cystoscopy. Dr. Daneshmand gave an overview of enhanced cystoscopy. He began discussing narrow band imaging (NBI), which works by filtering white light into specific light wavelengths that are absorbed by hemoglobin, providing enhanced visualization of capillary networks and mucosal morphology. It is not intended to replace histopathological sampling as a means of diagnosis. There have been several studies, mostly small, except for the study from Dr. Herr, which included over 400 patients, examining the specificity and sensitivity of NBI. The sensitivity and specificity in these studies ranged from 93%-100%, and 69%-85%, respectively. NBI alone enabled identification of 12%-27% additional tumors, as detailed in table 1.
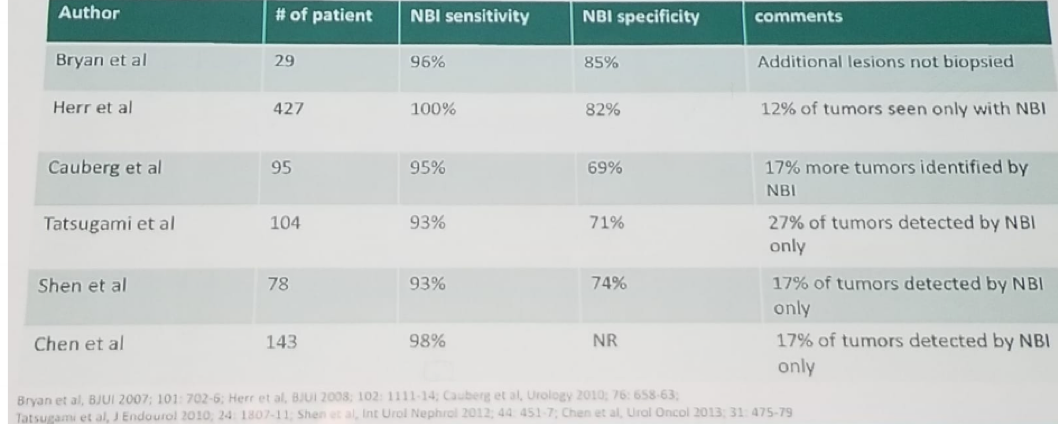
Table 1- NBI series demonstrating high specificity and sensitivity:
Next, blue light cystoscopy was explained by Dr. Daneshmand. This modality is used with an optical imaging agent (Cysview or Hexvis). In the US, Cysview is used with the Karl STORZ D- light C photodynamic diagnosis (PDD). Blue light cystoscopy has been shown to assist restaging following intravesical therapy. It has been extensively studied to investigate improvement in the detection of bladder tumors vs. white light cystoscopy. There are currently five multicenter phase- three trials in the US, Canada, and Europe assessing its impact. Over 1800 patients with known or suspected bladder cancer are enrolled in these trials. In a meta-analysis of 5 studies including more than 1300 patients, blue light cystoscopy reduced the rate of progression in bladder cancer to 6.8% compared to 10.7% in the standard white light cystoscopy patients, p=0.01. Additionally, it increased the time to progression. 1,2
Dr. Daneshmand concluded his brief talk comparing NBI to blue light cystoscopy, showing several advantages and disadvantages in each modality. NBI does not need the instillation of any agent, it is possible to perform transurethral resection of bladder tumor (TURBT) under NBI, it is cost-effective, and it increases the detection rate of tumors. In contrast, blue light cystoscopy has been extensively studied, it decreases recurrence and progression, has less inter-observer variability compared to NBI, and it detects carcinoma in situ (CIS) which is not visible with white light cystoscopy.
Next, Dr. Babjuck from the Czech Republic discussed the importance of the restaging TURBT. In a systematic review including 31 studies with over 8400 patients, it was demonstrated that after the initial TURBT the rate of residual tumor in Ta and T1 disease is 55%, and 51%, respectively. The upstaging rates were 0.4% and 8%, respectively. 3 Of these residual tumors, 86% were in the original tumor location site. The timing of the restaging TURBT is also an important and controversial question. A multicenter retrospective study including 242 patients from 10 centers demonstrated that the interval between the first TURBT and the second TURBT was associated with better recurrence-free survival, and progression-free survival when it was shorter than 42 days. 4
In T1 high-grade disease, a second TURBT is essential as it has a positive impact on recurrence-free survival, progression-free survival, and disease-specific survival only in the absence of muscle in the initial TURBT.5 Therefore, the quality of the initial resection is quite critical.
The European Association of Urology (EAU) guidelines state that a second TURBT must be performed after incomplete initial TURBT, or in case of doubt about the completeness of the TURBT. It should also be performed when there is no muscle in the specimen, except when the pathology shows Ta low grade and primary CIS. Lastly, in all T1 tumors, it should be performed as well. According to the EAU guidelines, the timing should be within 2-6 weeks from initial resection and must include resection of the primary tumor site.
Dr. Babjuck concluded his talk stating the required criteria for objective quality assessment of the TURBT. Our goal is for the patient to have low recurrence and progression rates. It has been shown that muscle presence in the initial TURBT and the surgeon’s experience predict early recurrence. 6 When a muscle is present in high-risk disease tumor persistence and T2 muscle-invasive disease is demonstrated in 75% and 15%, respectively. In contrast, when there is no muscle in the initial TURBT, 80% of tumor persists, and 45% of cases have T2 muscle-invasive disease! 7 Lastly, the surgeon should register his results for quality improvement. This registration should include the presence of muscle, the recurrence and progression rates, the results of a second TURBT and the positive margins rates.
Dr. Martinez-Pineiro was the next speaker, discussing the available treatment options of T1 high-grade disease. These include either upfront radical cystectomy or Bacillus Calmette–Guérin (BCG) intravesical instillations. A large study critically analyzed the treatment strategy for newly diagnosed high-grade T1 bladder tumors, involving more than 1000 patients, with a median follow-up of 69.6 months. The recurrence, progression, disease-specific survival and overall survival rates of patients who were treated with Bacillus Calmette–Guérin (BCG) intravesical instillations were 45.2% (23-74%), 23.1% (5-49%), 85.1% (77-100%), and 70.3% (60-95%), respectively.8 In contrast, when assessing the results of similar patients treated with radical cystectomy upfront, upstaging occurred in 35%, positive lymph nodes were demonstrated in 13.2%, disease-specific survival was 82.2%, and overall survival was 68.5%.
When comparing the major disadvantages of BCG to radical cystectomy, it is evident that BCG requires multiple reevaluations, with anesthesia, second TURBT, and associated hospital stay. Furthermore, BCG has known toxicities and adverse effects and can cause anxiety due to the know anticipation of radical cystectomy. On the other hand, radical cystectomy is major surgery, resulting in impotence, incontinence, delayed morbidity, and altered body image.
Dr. Martinez-Pineiro concluded his talk by addressing the most important question the urologist needs to answer in this regard: which patients would benefit from early upfront radical cystectomy? Patients who fail BCG will progress in 72.4% of the time. 9 Patients who recur less than six months from the TURBT will usually do worse, and further BCG maintenance will not improve their outcome, making them the ideal candidate for early radical cystectomy. Additionally, 82% of patients with the residual T1 disease after a second TURBT will progress and should also be referred for radical cystectomy. Lastly, patients with variant histology such as micropapillary, lymphoepithelial like differentiation, nested variant, and plasmacytoid variant have cancer-specific survival of 19% when treated with BCG and then radical cystectomy, compared to 72% in those treated only with early radical cystectomy.10
Dr. Kamat moved on to discuss the various definitions of BCG failure briefly.
BCG refractory disease is defined as a persistent high-grade disease at six months despite adequate BCG treatment. It also includes any stage/grade progression at three months after intravesical BCG.
BCG relapsing disease is defined as highest risk relapsing patients: within six months of last exposure to BCG.
BCG unresponsive is defined as BCG refractory and BCG relapsing within six months of the last exposure to BCG.
Dr. Konety was the last speaker of this long session discussing the treatment options for BCG unresponsive patients. The first option discussed is the FDA approved Valrubicin. This well-tolerated drug showed a 21% complete response rate, and 7% durable response at 30 months median follow-up in BCG refractory CIS patients, for which the FDA approved it for. Another option is Gemcitabine and Docetaxel. This combination was shown to produce a 16% treatment alteration in previously BCG treated patients. 11 Several other experimental potential options were only mentioned briefly. These include the CG0070 adenovirus expressing GM-CSF, and the ALR 803 recombinant fusion protein IL-15.
Dr. Konety concluded his brief talk, mentioning the association of BCG to PD-L1. High levels of PD-L1 have been shown to be present in BCG granulomas. The high PD-L1 expression has been demonstrated to be linked to BCG refractory disease, which occurs in 7% of pTa, 16% of pT1, and 45% of CIS patients.12 There are currently several ongoing checkpoint inhibitor trials assessing the role of Atezolizumb, Durvalumab, Pembrolizumab, Avelumab, and immune checkpoint inhibitor drug combinations in BCG refractory disease, and we all eagerly await these results.
Presented by: Ashish M. Kamat, MD Anderson Cancer Center, US
Co-Authors: Sia Daneshmand, Keck Medicine USC, US, Marek Babjuk, Prague, Czech Republic, Luis Martinez-Pineiro, Madrid, Spain, Dr. Badrinath R. Konety, University of Minnesota, US
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the Global Conference on Bladder Cancer 2018 - September 20-21, 2018 Madrid, Spain
References:
1. Gakis et al. Bladder Cancer July 2016
2. Kamat A et al. The bladder cancer journal April 2016
3. Cumberbatch MGK et al. Eur Urol 2018
4. Sumer B et al. BJU Int 2015
5. Gantero P et al. BJU Int 2016
6. Mariappan et al. BJU Int 2011
7. Herr et al. BJU Int 2008
8. Kulkarni G et al. Eur Urol 2010
9. Solsona et al. J Urol 2000
10. Kamat AM et al. J Urol 2006
11. Steinberg RL et al. Bladder Cancer 2015
12. Inman B et al. Cancer 2017


