(Urotoday.com) The newly formed Global Society of Rare Genitourinary Tumors (GSRGT) held its inaugural meeting, focusing on penile and testicular cancer. The session held Saturday, December 12th focused on testis cancer. In this session, the organizers convened a “Clinical Trials Corner” in which Dr. Craig Nichols presented an update on the SWOG S1823 study, a prospective observational cohort study assessing microRNA 371 for outcome prediction in patients with germ cell tumors.
Dr. Nichols began by highlighting data from a pilot study of miR-371. In a 2x2 analysis assessing patients with germ cell tumors with or without clinically confirmed germ cell malignancy (GCM), these pilot data demonstrated promising test characteristics: sensitivity of 96% (95% confidence interval 85-99%), the specificity of 100% (95% CI 96-100%, the positive predictive value of 100% (95% CI 92-100%), and negative predictive value of 98% (95% CI 92-100%).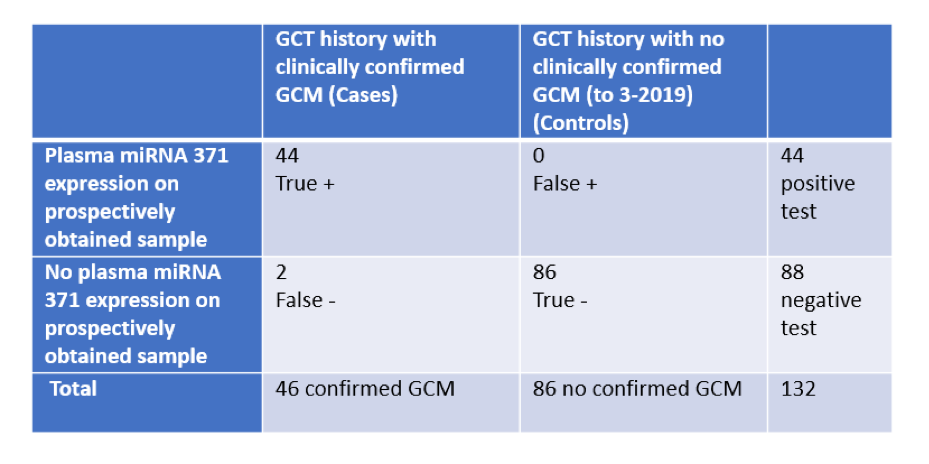
Utilizing area under the receiver operating curve to capture the diagnostic utility of miRNA-371, Dr. Nichols showed that miR371 out-performed CT scan and the conventionally used tumor markers (beta-HCG, LDH, and AFP) in both the overall cohort and those with moderate risk.
ROC Curves in All and Moderate Risk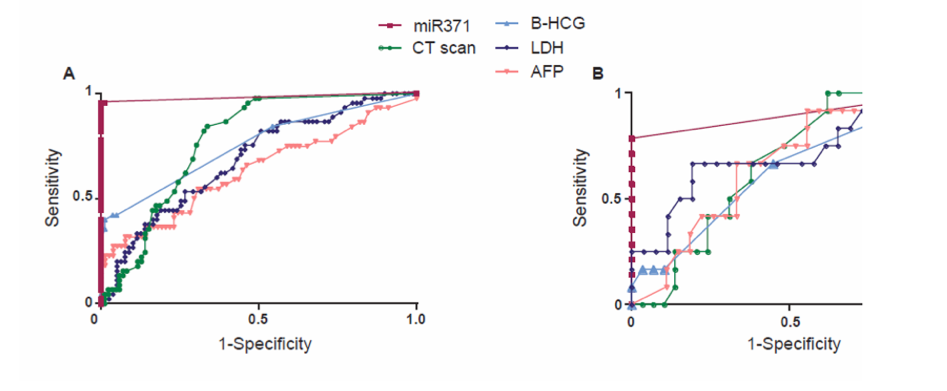
Given these promising data for miR371, Dr. Nichols highlighted the rationale for SWOG S1823, with the primary objective to estimate the positive predictive value within each of the early-stage testicular seminoma and non-seminoma groups using plasma miRNA 371 expression at relapse to detect germ cell malignancy. Secondary objectives relate to generating an associated biobank of serial liquid biospecimens for patients with annotated patient-level clinical data. For patients with high-risk of relapse and those with non-testicular primary tumors, this is being done in conjunction with the COG AGCT 1531 trial.
Currently, the trial is opening across the United States and Canada and is powered for 100 relapses in non-seminoma and 50 in seminoma among patients undergoing active surveillance. Efforts to coordinate with COG 1531, as well as similar studies in the Italian Study Group and Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP), may allow for data sharing. According to the SWOG website, the study is currently 4% accrued.
Dr. Nichols closed by discussing a number of global priorities in germ cell tumor clinical research:
-high fidelity clinical assessments including miR371, miR375, radiomics, and other miR approaches
-centralization of care for initial management and oversight given this uncommon disease
-improved cancer care delivery in lower resource jurisdictions
-improved survivorship, including reduction of over/under-treatment, costs, and long-term toxicity
-collaborations allowing for real-world data sharing
He suggested that salvage therapy and chemo-resistant disease remain low-impact areas of resource given decades of research with little observed benefit to targeted therapy and immunotherapy.
Presented by: Craig Nichols MD, FASCO, SWOG Executive Officer, Founder and Director Testicular Cancer Commons
Written by: Christopher J.D. Wallis, MD, Ph.D., Instructor in Urology, Vanderbilt University Medical Center, Nashville, Tennessee @WallisCJD on Twitter during the 1st Global Society of Rare Genitourinary Tumors Virtual Summit, December 11-12, 2020


