To do so, he utilized a case example of a 39-year-old patient who is healthy, without comorbidity, and was married with 2 children and working as a banker. The patient was a non-smoker but had a history of lung cancer in his mother and he had no sexual dysfunction. This man presented with an apparent increase in the size of the left testicle over 1.5 weeks. Ultrasound confirmed a 4cm vascular, hypoechoic lesion in the left testicle. Serum tumor markers were negative and testosterone was 8 nmol/L.
Following this diagnosis, the patient underwent staging with chest, abdomen, and pelvis CT scan which demonstrated no evidence of lymphadenopathy or metastases. Semen analysis demonstrated oligoasthenospermia though he underwent successful sperm cryopreservation followed by left orchiectomy and prosthesis placement. Histologic evaluation of the testicle noted a 4cm classical seminoma, stage pT1b.
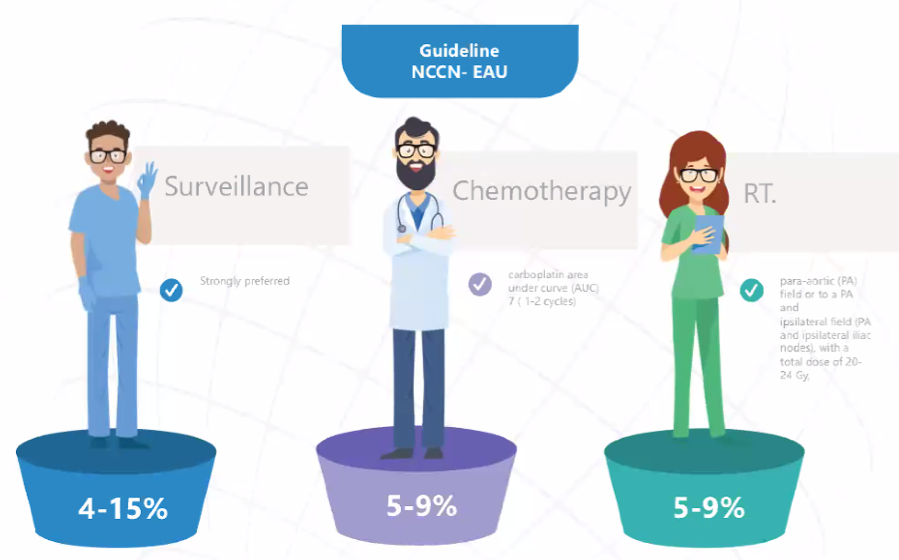
Highlighting National Comprehensive Cancer Network (NCCN) and European Association of Urology (EAU) guidelines offering surveillance, chemotherapy, and RT, Dr. Castiglione then discussed next steps including active surveillance with follow-up according to the NCCN guidelines, as well as testosterone replacement therapy given his hypogonadism.
Six months later, Dr. Castiglione described a surveillance CT scan identifying a 1.9cm para-aortic lymph node, with persistently negative markers, consistent with CS IIa seminoma.
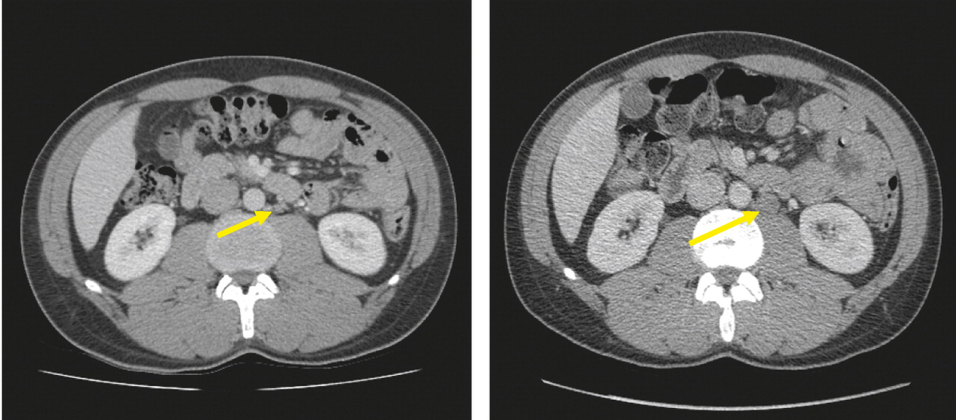
Now, this gentleman has good prognosis metastatic germ cell tumor, per IGCCCG criteria. Dr. Castiglione highlighted that there are a number of guideline supported treatment options including BEP x3, EP x4, or radiotherapy. In considering treatment options, Dr. Castiglione highlighted the importance of considering the side effects of therapy including secondary cancer, gastrointestinal toxicity, and cardiovascular disease attributable to radiotherapy and chemotherapy.
He highlighted newer approaches including the potential to de-escalate chemotherapy on the basis of use of interim PET-CT, as highlighted in the SEMITEP trial utilizing EP x2 followed by either carboplatin or a further 2 cycles of EP on the basis of the PET-CT results. Early data from this trial demonstrated similar progression-free survival. A second approach uses carboplatin AUC-10 guided by PET-CT.
Further, while more experimental, there may be a role for retroperitoneal lymph node dissection (RPLND) as highlighted in a number of relatively small case series. He highlighted two ongoing prospective trials of RPLND in seminoma, the SEMS trial, and PRIMETEST trial.
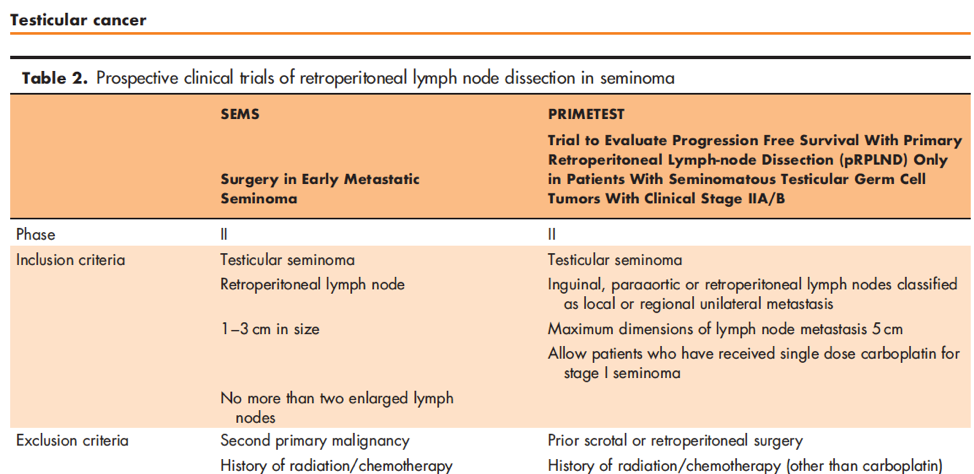
Based on data presented at ASCO-GU 2019, an interim analysis of PRIMETEST among 22 patients showed 17/22 free of recurrence with only one grade 3 complication, ureteral stricture requiring ileal ureter substitution. Notably, 4 of 5 recurrences were out-of-field.
In closing, he highlighted that the patient, in this case, the patient opted for BEP x3 cycles.
Presented by: Fabio Castiglione, MD, PhD, FEBU, FECSM, San Raffaele Scientific Institute, Milan, Italy
Written by: Christopher J.D. Wallis, MD, PhD, FRCSC, Instructor in Urology, Vanderbilt University Medical Center, Nashville, Tennessee Contact: @WallisCJD on Twitter during the 1st Global Society of Rare Genitourinary Tumors Virtual Summit, December 11-12, 2020


